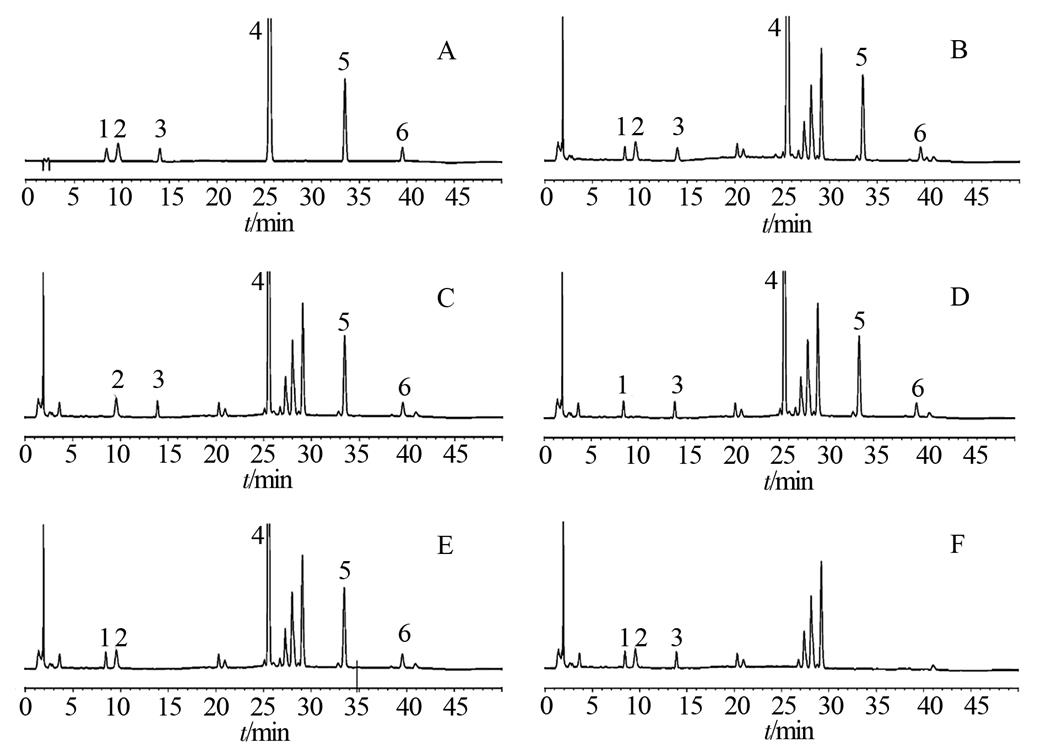
图1 高效液相色谱图
邓俊杰
(绍兴市食品药品检验研究院,浙江 绍兴 312088)
摘要:目的建立HPLC双波长法同时测定炎热清片中龙胆苦苷、栀子苷、芒果苷、黄芩苷、黄芩素和汉黄芩素6种成分的含量。方法采用Agilent TC-C18色谱柱(4.6 mm×250 mm,5 μm),流动相为甲醇∶0.2%磷酸水溶液(梯度洗脱),柱温为35 ℃,流速为1.0 mL·min-1,检测波长0~20 min为254 nm,同时检测龙胆苦苷、栀子苷、芒果苷,20~50 min为280 nm,同时检测黄芩苷、黄芩素、汉黄芩素。结果龙胆苦苷、栀子苷、芒果苷、黄芩苷、黄芩素、汉黄芩素分别在18.92~189.2 ng(r=0.999 9),107.44~1 074.4 ng(r=0.999 9),9.82~98.2 ng(r=0.999 9),767.68~7 676.8 ng(r=1.000 0),100.94~ 1 009.4 ng(r=0.999 9),49.84~498.4 ng(r=0.999 9)线性良好,平均回收率(n=9)分别为98.31%,98.35%,98.40%,98.03%,98.46%,98.24%,RSD分别为0.4%,0.3%,0.3%,0.4%,0.4%,0.3%。结论该方法灵敏、简便、准确,可用于炎热清片的质量控制。
关键词:炎热清片;龙胆苦苷;栀子苷;芒果苷;黄芩苷;黄芩素;汉黄芩素;高效液相色谱法
炎热清片现行标准为国家药品监督管理局国家标准YBZ13632005-2011Z-5,处方由龙胆、栀子、知母、黄芩、玄参、石膏、柴胡、薄荷脑8味中药组成,具有解表清里、清热解毒的功效。临床用于呼吸道炎、支气管炎、肺炎、急性扁桃体炎,也可用于泌尿系统感染、胆道感染[1-3]。
目前,国家标准YBZ13632005-2011Z-5中只以黄芩苷1种成分作为指标考察制剂质量,既缺少对以黄芩苷、黄芩素和汉黄芩素为代表的总黄酮含量的研究,也缺少对处方中其他组分药材的质控指标,整体质量的控制尚显不足。本实验采用HPLC双波长法同时测定龙胆主要成分龙胆苦苷,栀子主要成分栀子苷,知母主要成分芒果苷,黄芩主要成分黄芩苷、黄芩素、汉黄芩素,从1种成分的检测到同时检测6种成分,较大幅度地提升整体质量标准,经验证该方法灵敏、简便、准确,可用于炎热清片的质量控制[4-15]。
Agilent 1260高效液相色谱仪(美国Agilent公司);UV-2550型紫外可见分光光度计(岛津仪器有限公司);XPE 105电子天平(Mettler Toledo梅特勒-托利多公司);FESCO17微量离心机(Termo赛默飞世尔科技公司);KQ-300DE型数控超声波清洗器(昆山市超声波仪器有限公司)。
龙胆苦苷(批号:110770-201314;含量以100.0%计),栀子苷(批号:110749-201316;含量以97.5%计),芒果苷(批号:111607-201503;含量以98.4%计),黄芩苷(批号:110715-201318;含量以93.3%计),黄芩素(批号:111595-201607;含量以98.5%计),汉黄芩素(批号:111514-201605;含量以100.0%计)均购自中国食品药品检定研究院;乙腈(色谱纯,美国天地试剂公司);磷酸(分析纯,国药集团化学试剂有限公司);超纯水;炎热清片(吉林天药本草堂制药有限公司,批号分别为150304,160509,160704)。
色谱柱:Agilent TC-C18柱(4.6 mm×250 mm,5 μm);流动相:甲醇(A)-0.2%磷酸水溶液(B),梯度洗脱(0~15 min,12%A;15~ 42 min,12%→15%A;42~50 min,50%→12%A);柱温:35 ℃;流速:1.0 mL·min-1;检测波长:0~20 min为254 nm,20~50 min为280 nm,进样量:10 μL。
2.2.1 对照品溶液的制备 精密称取①龙胆苦苷对照品9.46 mg,置于100 mL量瓶中;②栀子苷对照品11.02 mg,置于20 mL量瓶中;③芒果苷对照品12.47mg,置于250 mL量瓶中;④黄芩素对照品12.81 mg,置于25 mL量瓶中;⑤汉黄芩素对照品12.46 mg,置于50 mL量瓶中,分别加70%甲醇溶解并稀释至刻度,作为对照品贮备液。再精密称取黄芩苷对照品20.57 mg,置于50 mL量瓶中,加入①~⑤号对照品储备液各5.0 mL,加70%甲醇稀释至刻度,制成混合对照品溶液,龙胆苦苷、栀子苷、芒果苷、黄芩苷、黄芩素、汉黄芩素的含量分别为9.46,53.72,4.91,383.84,50.47,24.92 μg·mL-1(含量均已计算相应纯度)。
2.2.2 供试品溶液的制备 取本品适量,除去包衣,研细,混匀,精密称取0.5 g,置50 mL量瓶中,加入70%甲醇40 mL,超声处理30 min,取出,放冷至室温,用70%甲醇稀释至刻度,摇匀,离心,取续滤液,即得。
2.2.3 阴性供试品溶液制备 按处方组成及生产工艺,分别制备缺龙胆、栀子、知母、黄芩的4种阴性供试品,再按供试品溶液制备法制得阴性供试品溶液。
分别取对照品溶液、供试品溶液和4种阴性供试品溶液各10 μL,按“2.1”项下色谱条件注入高效液相色谱仪,记录色谱图。结果供试品色谱图中显示与对照品相同保留时间的色谱峰,且各色谱峰分离度均>1.5,阴性供试品溶液在此保留时间无色谱峰出现,表明处方中其他成分对结果无干扰,该方法系统适应性良好,结果见图1。

图1 高效液相色谱图
A-混合对照品;B-供试品;C-缺龙胆阴性供试品;D-缺栀子阴性供试品;E-缺知母阴性供试品;F-缺黄芩阴性供试品;1-龙胆苦苷;2-栀子苷;3-芒果苷;4-黄芩苷;5-黄芩素;6-汉黄芩素。
Fig. 1 HPLC chromatogram
A-mixed reference substances; B-sample; C-negative sample without Gentiance Radix et Rhizoma; D-negative sample without Gardeniae Fructus; E-negative sample without Anemarrhenae Rhizoma; F-negative sample without Scutellariae Radix; 1-gentiopicroside; 2-geniposide; 3-mangiferin; 4-baicalin; 5-baicalein; 6-wogonin.
分别精密量取混合品对照品溶液2,4,8,12,16,20 μL,按“2.1”项下色谱条件进样,测定峰而积。以峰面积为纵坐标(Y),进样量为横坐标(X)绘制标准曲线,计算得线性回归方程,结果见表1。
表1 6种成分的线性回归方程
Tab. 1 The regression curve of six components

取“2.2.1”项下对照品溶液,按“2.1”项下色谱条件,每次进样10 μL,连续进样6次,记录色谱峰面积,结果龙胆苦苷、栀子苷、芒果苷、黄芩苷、黄芩素、汉黄芩素峰面积的RSD分别为0.3%,0.1%,0.3%,0.2%,0.2%,0.4%,表明仪器精密度良好。
取6份同一批号供试品粉末(批号:160704),以供试品溶液制备法制备,按“2.1”项下色谱条件测定并计算,结果龙胆苦苷、栀子苷、芒果苷、黄芩苷、黄芩素、汉黄芩素的含量分别为0.779,5.41,0.491,41.6,5.01,2.53 mg·g-1,RSD分别为0.3%,0.1%,0.5%,0.4%,0.3%,0.2%,表明重复性较好。
取同一供试品溶液,分别在0,8,12,24,48 h,按“2.1”项下色谱条件测定并计算,结果龙胆苦苷、栀子苷、芒果苷、黄芩苷、黄芩素、汉黄芩素在不同时间点测得含量的RSD分别为0.3%,0.5%,0.6%,0.4%,0.5%,0.4%,表明供试品溶液在48 h内稳定。
2.8.1 加标用对照品溶液的制备 精密称取①龙胆苦苷对照品20.24 mg;②芒果苷对照品12.72 mg,③汉黄芩素对照品64.32 mg分别置于10 mL量瓶中,加70%甲醇溶解并稀释至刻度,作为①、②、③储备液。再精密称取栀子苷对照品13.97 mg,黄芩苷对照品112.56 mg,黄芩素对照品12.87 mg,置于同一20 mL量瓶中,加入①、②、③储备液各1.0 mL,加70%甲醇溶解并稀释至刻度,制成加标用对照品溶液,龙胆苦苷、栀子苷、芒果苷、黄芩苷、黄芩素、汉黄芩素的含量分别为0.101 2,0.681,0.062 6,5.251,0.634,0.321 6 mg·mL-1。
2.8.2 加样回收率测定 取9份已知含量的同一批号供试品粉末(批号:160704),每份约0.25 g,精密称定,分置具塞锥形瓶中,3份为1组,分别加入加标用对照品溶液1.0,2.0,3.0 mL(约相当于样品含量的50%,100%,150%),以供试品溶液制备法制备,按上述色谱条件测定,并计算回收率,结果见表2。3组不同对照品加入量(n=9)间的平均回收率,龙胆苦苷、栀子苷、芒果苷、黄芩苷、黄芩素、汉黄芩素分别为98.31%,98.35%,98.40%,98.03%,98.46%,98.24%,RSD分别为0.4%,0.3%,0.3%,0.4%,0.4%,0.3%。
取3批供试品(150304,160509,160704)粉末各约0.5 g,精密称定,以供试品溶液制备法制备,按“2.1”项下色谱条件测定并计算含量,结果见表3。
表2 加样回收率试验测定结果(n=9)
Tab. 2 Results of recovery test(n=9)
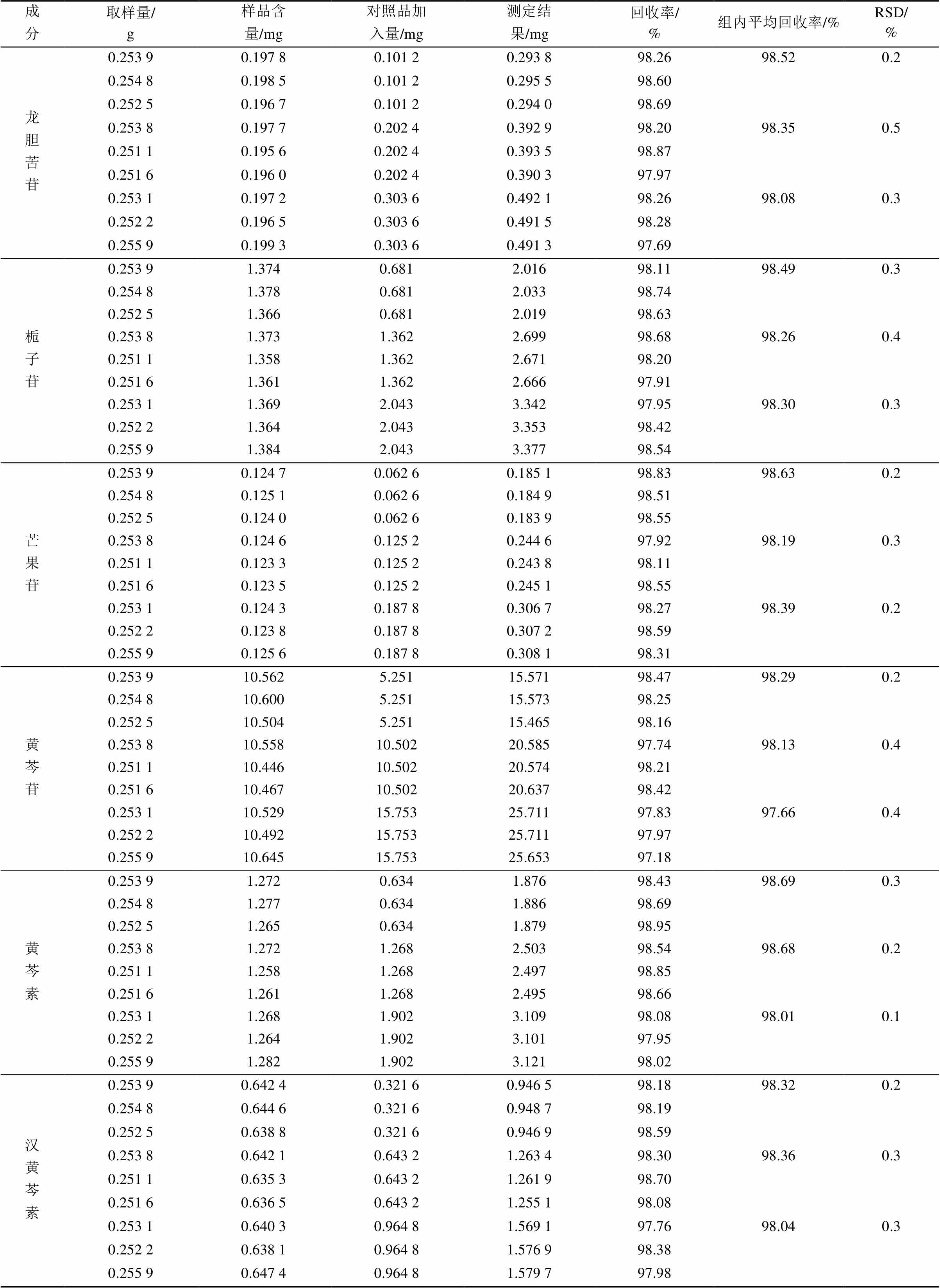
表3 供试品含量测定结果(n=3)
Tab. 3 Results of sample determination(n=3) mg·g-1

参考中国药典2015年版一部和相关文献,确定各组分常用流动相的选择如下:龙胆苦苷为甲醇∶水(25∶75),栀子苷为乙腈∶水(15∶85),芒果苷为乙腈∶0.2%冰醋酸(15∶85),黄芩苷、黄芩素和汉黄芩素为甲醇∶0.2%磷酸(47∶53)。故流动相的选择可以分为2组:龙胆苦苷、栀子苷、芒果苷3者为1组,黄芩苷、黄芩素、汉黄芩素3者为1组,2组流动相的洗脱能力相差较大,故采用梯度洗脱的方法。
比较甲醇和乙腈作为有机相的分离效果,由于龙胆苦苷、栀子苷、芒果苷3者结构类似,甲醇分离效果优于乙腈,最终以甲醇作为有机相,同时在水相中添加0.2%的磷酸,有效改善各组分的峰型。
取龙胆苦苷、栀子苷、芒果苷、黄芩苷、黄芩素和汉黄芩素对照品适量,分别加 70%甲醇溶解后,使用紫外可见分光光度计分别测定紫外光谱图,结果各组分的最大吸收波长分别为:龙胆苦苷254,270 nm,栀子苷238 nm,芒果苷241,258,318 nm,黄芩苷213,278,315 nm,黄芩素214,275,322 nm,汉黄芩素199,275 nm。流动相中甲醇在190~220 nm处有吸收,考虑在避免干扰的同时,使各组分均有较高响应值,最终确定检测波长0~20 min为254 nm,20~50 min为280 nm。
取用甲醇、乙醇、70%甲醇作为提取溶剂,分别制备供试品溶液,结果发现用70%甲醇作为溶剂提取效果好,杂质峰较少,故选用70%甲醇作为提取溶剂。
比较了超声和回流2种提取方法,发现提取效果差异小,相同提取时间下,含量基本相同,考虑提取过程中,超声法比回流法溶剂挥发量少,结果重复性好,故选择超声提取法。
比较了超声时间20,30,45,60 min对结果的影响,结果在超声时间20 min时,黄芩苷含量偏低,未能完全提取,30,45,60 min下,各组分含量基本相同,故选择超声时间为30 min。
references
[1] 中华人民共和国卫生部药品标准中药成方制剂第八册[S]. 1993: 101.
[2] YBZ13632005-2011Z-5, 国家食品药品监督管理局国家药品标准[S]. 2012.
[3] 中国药典. 一部[S]. 2015: 96, 212-213, 248, 301-302.
[4] GUAN X Y,CHEN X L,WANG S H, et al. Simultaneous determination of 4 effective components in Fufang Yuxingcao tablets by HPLC [J].Chin J Mod Appl Pharm(中国现代应用药学), 2017, 34(9): 1300-1303.
[5] WANG W Y, MAO J H, YU L, et al. Quality evaluation of decoction pieces of Gardeniae Fructus in market [J]. Chin J Mod Appl Pharm(中国现代应用药学), 2017, 34(5): 681-685.
[6] WANG J P, CHAI J, LYU L F. Simultaneous determination of aloe-emoidin, baicalin, obacunone and crocinⅠin Sijisanhuang tablets by HPLC [J]. Chin J Mod Appl Pharm(中国现代应用药学), 2016, 33(2): 199-203.
[7] ZHOU Z H, WEI J N, ZHAO L L. Multi-index comprehensive evaluation of Radix Scutellariae [J]. Mod Chin Med(中国现代中药), 2015, 17(1): 31-38.
[8] FU L, SHI J H. Antibacterial activityin vitroand anti-inflammatory effectsin vivoof baicalein [J]. China Pharma(中国药房), 2014, 25(23): 2136-2138.
[9] ZHAO Y W, YU M, SI X L, et al. TLC indentification and content determination of gentiopicroside in Yan Reqing tablet [J]. Chin J Exp Tradit Med Form(中国实验方剂学杂志), 2013, 19(3): 118-120.
[10] NING K X, HUANG Y P. Determination of geniposide, baicalin and wogonin in Yanreqing tablets by HPLC [J]. China Pharm(中国药业), 2015, 24(16): 89-91.
[11] ZHOU P, SONG Q, MA S. Simultaneous determination of geniposide, peoniflorin, baicalin, baicalein and wogonin in Xiao’er Chiqiao Qingre granules by HPLC [J]. Chin Tradit Pat Med(中成药), 2013, 35(8): 1693-1696.
[12] ZHAO L, WANG X X, LV L, et al. Determination of baicalin, wogonoside, baicalein, wogonin in Qinggan Sanjie granules by HPLC [J]. Chin Tradit Pat Med(中成药), 2014, 36(2): 313-318.
[13] WANG W N, ZHOU Q, LI H. Simultaneous determination of gentiopicrin and baicalin in Erlong capsules by HPLC [J]. Chin Pharm Aff(中国药事), 2013, 27(7): 732-734.
[14] ZHANG X F, LUO G F, WANG Y. Simultaneous determination of swertiamarin, gentiopicroside and mangiferin in Swertia Franchetiana by HPLC [J]. Chin J Exp Tradit Med Form(中国实验方剂学杂志), 2014, 20(12): 61-64.
[15] ZHANG H M, CHANG S, CUI B J, et al. Simultaneous determination of 6 kinds of components in Yindan Pinggan capsules by HPLC [J]. China Pharm (中国药房), 2017, 28(9): 1239-1242.
Simultaneous Determination of Six Components in Yanreqing Tablets by Double-wavelength HPLC
DENG Junjie
(Shaoxing Institute for Food and Drug Control, Shaoxing 312088, China)
ABSTRACT:OBJECTIVETo establish a new double-wavelength HPLC method for simultaneous determination of 6 main components in Yanreqing tablets.METHODSThe method was performed on an Agilent TC-C18column(4.6 mm×250 mm, 5 μm). The mobile phase was consisited of methanol-0.2% phosphoric acid by gradient elution at 35 ℃ column temperature. The flow rate was 1.0 mL·min-1. The detection wavelength was 254 nm for gentiopicroside, geniposide , mangiferin at 0-20 min, and 280 nm for baicalin, baicalein, wogonin at 20-50 min.RESULTSGentiopicroside, geniposide, mangiferin, baicalin, baicalein, wogonin showed a good linear relationship in the range of 18.92-189.2 ng(r=0.999 9), 107.44-1 074.4 ng(r=0.999 9), 9.82-98.2 ng(r=0.999 9), 767.68-7 676.8 ng(r=1.000 0), 100.94-1 009.4 ng(r=0.999 9), 49.84-498.4 ng(r=0.999 9), respectively. The average recoveries were 98.31%, 98.35%, 98.40%, 98.03%, 98.46%, 98.24%, the RSD were 0.4%, 0.3%, 0.3%, 0.4%, 0.4%, 0.3%.CONCLUSIONThe method is sensitive, simple, accurate, and suitable for the quality control of Yanreqing tablets.
KEY WORDS:Yanreqing tablets; gentiopicroside; geniposide; mangiferin; baicalin; baicalein; wogonin; HPLC
中图分类号:R927.2
文献标志码:B
文章编号:1007-7693(2018)09-1329-04
DOI:10.13748/j.cnki.issn1007-7693.2018.09.013
引用本文:邓俊杰. HPLC双波长法同时测定炎热清片中6种有效成分的含量[J]. 中国现代应用药学, 2018, 35(9): 1329-1332.
收稿日期:2017-11-08
作者简介:邓俊杰,男,硕士 Tel: (0575)89101355 E-mail: 79761822@qq.com
(本文责编:曹粤锋)