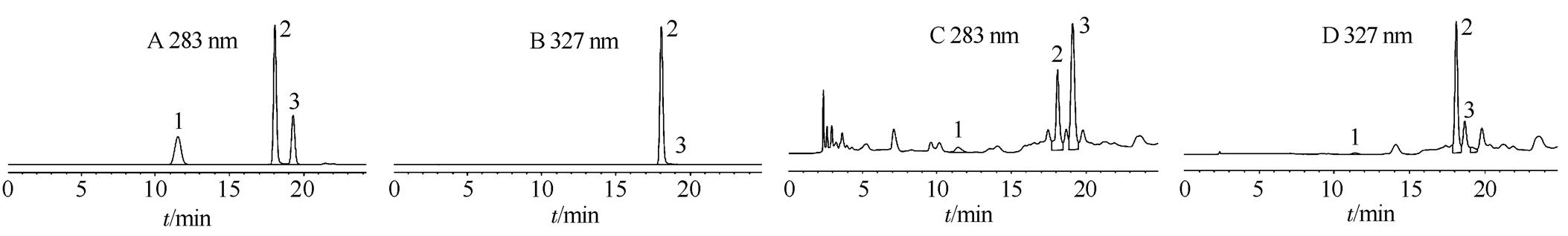
图1 3种酚酸对照品(A,B)和样品(C,D)HPLC色谱图
张雨林,詹济华,谭洋,李玲*,裴刚*
(湖南中医药大学药学院,长沙 410208)
摘要:目的建立同时测定沅江产芦笋中原儿茶酸、绿原酸、香草酸、柚皮苷、芦丁、槲皮素、木犀草素、山奈酚、芹菜素含量的HPLC。方法采用Diamonsil C18色谱柱(250 mm×4.6 mm,5 μm),以甲醇-0.1 %甲酸水溶液为流动相,梯度洗脱,双波长检测(酚酸:283,327 nm;黄酮:283,335 nm),流速1 mL·min-1,柱温35 ℃,进样量为10 μL。结果3种酚酸及6种黄酮分别在25 min和50 min内分离,线性范围为14.8~500 μg·mL-1(r=0.999 0~0.999 7),检测限0.006 3~ 0.053 9 μg·mL-1,定量限0.021 1~0.179 7 μg·mL-1,加样回收率为98.73%~102.44%,RSD为1.27%~2.20%(n=6)。结论该方法准确灵敏、重复性好,可用于检测芦笋中9种成分含量。
关键词:芦笋;酚酸;黄酮;高效液相色谱法
芦笋[phragmites communis(Cav.) Trin. ex Steud]是禾本科芦苇属植物芦苇[Phragmites communis(Cav.) Trin.]的幼芽,又名南荻、荻笋,在洞庭腹地的沅江地区坐拥45万亩的生长区域。其中含有丰富的黄酮[1]、氨基酸[2]、矿质元素[3]、膳食纤维等活性成分[4],具有抗菌、抗氧化、提高免疫力、保肝、降血脂、抗肿瘤等药理作用[5-6]。芦笋资源丰富,天然野生,且独具风味、功效繁多,被誉为“洞庭虫草”[7]。目前对于该植物的应用多限于其成熟植株芦苇,用于造纸、防汛、固堤、编席、作药材等领域[3],其可食用嫩芽芦笋仅作为民间菜肴食用,而其化学成分的研究大部分是基于对成熟芦苇根、茎以及叶的成分研究[5-6],对于芦苇可食用幼芽芦笋的有效成分研究还极少涉及。现已有部分研究表明芦笋中含有黄酮、酚酸类成分[8-9],而黄酮和酚酸均具有一定的药理活性[10-11],黄酮更是如今活性成分研究的热门之一。因此本研究对沅江产芦笋中黄酮、酚酸类活性成分进行含量测定,从而分析洞庭湖区河滩野生芦苇嫩茎的营养价值和开发前景,为芦笋作为药用、保健产品资源的开发提供参考。
芦笋于2016年4月采自湖南省沅江市,经湖南中医药大学中药教研室肖锦仁副教授鉴定为禾本科芦苇属植物芦苇[Phragmites communis(Cav.) Trin.]的嫩芽,标本现保存于湖南中医药大学中药化学教研室,编号:20160411。甲醇为色谱纯(TEDIA公司,批号:17045110);双蒸水为自制;其余试剂均为分析纯。对照品柚皮苷(批号:110722-201610)、芦丁(批号:100080-201107)、木犀草素(批号:111520-201504)、山奈酚(批号:110861-201405)、原儿茶酸(批号:0809-9201)购自中国药品生物制品检定所,纯度均>95%;芹菜素(上海金穗生物科技有限公,批号:20161228;纯度>98%);槲皮素、香草酸、绿原酸为自制对照品,纯度>95%。
AL-204型电子分析天平(梅特勒托利多仪器公司);超声波清洗机(宁波新芝生物科技股份有限公司);LH-20岛津高效液相色谱仪(包括二元泵系列溶剂传输单元,Chromatopac色谱工作站)。
色谱柱:Diamonsil钻石C18(250 mm×4.6 mm,5 μm);流动相:甲醇(A)-0.1%甲酸水溶液(B),梯度洗脱(①酚酸:0~10 min,84% B;10~25 min,66% B;②黄酮:0~15 min,72%→57% B,15~25 min,57% B,25~45 min,50%→35% B,45~50 min,35%→72% B);流速:1 mL·min;柱温:35 ℃;进样体积:10 μL;检测波长:①酚酸为283,327 nm;②黄酮为283,335 nm。
HPLC色谱图见图1~2。在上述色谱条件下,各成分可分别在25 min和50 min内得到分离,且目标峰形尖锐,对称性好,理塔板数≥5 000。

图1 3种酚酸对照品(A,B)和样品(C,D)HPLC色谱图
1-原儿茶酸;2-绿原酸;3-香草酸。
Fig.1 HPLC chromatogram of 3 phenolic acid references substance(A, B) and sample(C, D)
1-protocatechuic acid; 2-chlorogenic acid; 3-vanillic acid.

图2 6种黄酮对照品(A,B)和样品(C,D)HPLC色谱图
1-柚皮苷;2-芦丁;3-槲皮素;4-木犀草素;5-山奈酚;6-芹菜素。
Fig. 2 HPLC chromatogram of 6 flavonoids reference substance(A, B) and sample(C, D)
1-naringin; 2-rutin; 3-quercetin; 4-luteolin; 5-kaempferol; 6-apigenin.
2.2.1 供试品溶液的制备 芦笋供试品的制备:芦笋60 ℃干燥,打粉,过四号筛。供试品一:取10 g芦笋粉末使用60%乙醇1 000 mL超声提取1 h,提取液60 ℃减压回流浓缩至50 mL;供试品二:取20 g芦笋粉末使用70%乙醇400 mL水浴加热回流提取1 h,重复3次,浓缩成浸膏共4.5 g,精密称取500 mg浸膏溶解于25 mL甲醇中。溶液均过0.45 μm微孔滤膜,4 ℃保存备用。
2.2.2 对照品溶液的制备 精密称取适量原儿茶酸、绿原酸、香草酸对照品置于容量瓶中,加入色谱甲醇定容至刻度,得到浓度分别为0.475,0.500,0.500 mg·mL-1的混合对照品1;精密称取适量柚皮苷、芦丁、槲皮素、木犀草素、山奈酚、芹菜素对照品置于量瓶中,加入色谱甲醇定容至刻度,得到各样品浓度为0.200 mg·mL-1的混合对照品2。溶液均过0.45 μm微孔滤膜,于4 ℃保存备用。
2.3.1 线性关系考察及检测限、定量限测定 使用二倍稀释法将对照品溶液稀释至初始浓度的1,0.5,0.25,0.125,0.062 5,0.031 25倍,取各浓度对照品溶液分别进样10mL,以峰面积为纵坐标(Y),质量浓度(X)为横坐标,绘制标准曲线,计算标准曲线的回归方程及相关系数,结果见表1。将配制的混合对照品储备液逐步稀释,在信噪比为3/1时所对应的化合物浓度即检测限(LOD),信噪比为10/1时所对应的化合物浓度即定量限(LOQ),结果见表 1。由表1可知,所测9种成分对照品浓度和相应峰面积呈良好线性关系;各个成分的检测限为0.006 34~0.053 9mg·mL-1,定量限为 0.021 1~0.179 7mg·mL-1,表明此方法灵敏度较高。
2.3.2 仪器精密度试验 取“2.2.2”项下对照品溶液,按“1.3.3”项下色谱条件重复进样6次,每次10 μL,测得原儿茶酸、绿原酸、香草酸、柚皮苷、芦丁、槲皮素、木犀草素、山奈酚、芹菜素的峰面积 RSD分别为1.38%,1.44%,2.39%,2.32%,1.89%,2.45%,2.32%,1.77%,2.78%,表明仪器精密度良好。
2.3.3 重复性试验 取6份芦笋浸膏,每份2.0 g,精密称定,按“2.2.1”项下方法制备供试品溶液,按“1.3.3”项下色谱条件重复进样6次,每次10 μL,测得9种成分的峰面积RSD分别为1.78%,2.11%,1.77%,0.63%,1.69%,2.21%,1.44%,0.98%,2.45%,表明该测定方法的重复性良好。
2.3.4 稳定性试验 取“2.2.1”项下的供试品溶液,按“1.3.3”项下的色谱条件,分别于0,2,4,8,12,24 h 进样分析,测得9种成分的峰面积RSD分别为0.78%,0.78%,1.98%,0.58%,0.96%,1.48%,1.59%,0.78%,1.25%,证明该供试品在24 h内稳定性良好。
2.3.5 回收率试验 吸取“2.2.1”中供试品一溶液1 mL,平行6份;称取“2.2.1”中供试品二浸膏20 mg,5 mL甲醇溶解,平行6份,按照药材样品量的50%,100%,150%加入相应标准品溶液,分别进样测定分析,计算回收率,得到各成分的加标回收率为98.73%~102.44%,且RSD值均<3%,说明本法具有良好的回收率,结果见表2。
2.3.6 供试品的含量测定 使用已制备供试品一、供试品二按“1.3.3”项下色谱条件进行检测,每份样品重复进样3次测定分析,根据表1中的线性方程得出样品中各个成分的含量,见表3。
表1 9种成分的线性关系、检测限和定量限
Tab. 1 Linear regression data, LOD, and LOQ of nine components

表 2 芦笋中9种成分加样回收率试验(n=6)
Tab. 2 The recovery test of nine components inPhragmites communis(n=6)

3.1.1 提取条件的选择 酚酸与黄酮在甲醇中溶解度不一样,且酚酸存在热不稳定性,因此采用不同方法提取,参考文献研究[12],供试品一采用60%乙醇作为提取溶剂,料液比为1∶100,使用超声提取法提取1 h,且使用减压回流法在60 ℃中浓缩,以供原儿茶酸、绿原酸、香草酸3种酚酸检测;供试品二采用70%乙醇作为提取溶剂,料液比为1∶20,通过水浴回流加热法反复提取3次,每次1 h,以供柚皮苷、芦丁、槲皮素、木犀草素、山奈酚、芹菜素6种黄酮检测。
3.1.2 检测波长的选择 查阅药典[13]及结合检测情况得到不同成分的最大吸收波长,其中香草酸、原儿茶酸、柚皮苷在283 nm处有最大吸收峰,绿原酸在327 nm处有最大吸收峰,芦丁、槲皮素、木犀草素、山奈酚、芹菜素335 nm处有最大吸收峰。综合考虑可分为3个波长进行检测,即283,327和335 nm。
3.1.3 流动相的选择 本试验分别使用甲醇-水、甲醇-甲酸水溶液、乙腈-甲酸水溶液等不同体系检测供试品,发现纯水无法实现酚类成分无拖尾现象的完全分离,乙腈也无法满足供试品的良好分离,因此选择0.1%甲酸溶液为水相,甲醇为有机相。通过尝试不同的梯度洗脱条件,最后确定本试验中的梯度洗脱程序。
本实验使用HPLC建立了同时检测包括3种酚酸、6种黄酮类成分在内的9种成分的方法,检测结果中芦笋中9种成分的含量为在87.29~ 1 340.37 μg·g-1,其中香草酸的含量最高,达到了0.134%。该方法中,各成分均有较宽的线性范围和良好的线性关系,且精密度、重复性、稳定性及加标回收率RSD值均<3%,说明该方法具有稳定、准确,定量限、检测限低,灵敏快捷等优点。
表3 芦笋中9种成分含量测定结果(n=3)
Tab. 3 The contents of nine components inPhragmites communis(n=3)
各项研究发现,原儿茶酸、绿原酸、香草酸均有抗菌[14]、抗炎、抗氧化[15]的作用,其中原儿茶酸、绿原酸具有保护肝脏、抗肥胖[16]、抗肿瘤[17-18]等药理作用[19-21]。黄酮类化合物更是近年来国内外研究热点,研究发现其具有抗氧化、抗菌抗炎、抗病毒、保护肝脏、抗糖尿病、抗心血管疾病等诸多功效[22]。例如槲皮素、芦丁等在胰岛素抵抗、心脏保护和预防血脂升高方面就有良好的疗效[23-24]。而且黄酮类化合物以其高效低毒的特性在肿瘤治疗上得到广泛的关注和研究,其中就包括柚皮苷[25]、芦丁[26]、槲皮素、木犀草素、芹菜素、山奈酚等[27]。综合说明本方法测定的芦笋中的数种成分有一定药理活性,初步确定了芦笋药效作用的物质基础,在芦笋的质量控制和药用、保健等功能性产品的研究开发中具有一定的参考价值。
References
[1] SHAO R, GUO H B, XU W, et al. Progress in the study of active substances from reed [J]. Chin J Biochem Pharm(中国生化药物杂志), 2011, 32(2): 167-168.
[2] YANG H L, CHEN G C. Analysis of nutrient compositions ofParagmites Comunisin different habitast [J]. Acta Prataculturae Sinica (草业学报), 1994, 3(1): 1-6.
[3] XIAO J J, ZHAO L Y, SHI X B, et al. Analysis of nutrients and heavy metals in tender stem ofTriarrhena lutarioripariafrom the Dongting lake area [J]. Food Sci(食品科学), 2015, 36(12): 104-107.
[4] YU X H, ZHU X M, LI F W, et al. Antioxidant properties of flavonoids extracted from leaves ofPhragmites communisTrin.in vivoandin vitro[J]. Food Sci(食品科学), 2015, 36(1): 209-213.
[5] GAN Q, LU F, HOU E T, et al. Research situation ofPhragmites communisTrin [J]. Pharm J Anhui Agr Sci(安徽农业科学), 2010, 38(15): 7878-7879.
[6] ZHAO X X, TAN C Y, MENG F T, et al. The research progress on chemical constituent and biological activity of phragmites australis [J]. Fine Spcl Chem(精细与专用化学品), 2013, 21(1): 20-22.
[7] 喻亚飞. 荻香润肤霜的药学研究[D]. 湖南中医药大学, 2016.
[8] WU L, GONG S. Purification of total flavonoids fromLysimachia fortuneiMaxim by macroporous absorption resins and ultrasonic wave [J]. Pharm Today(今日药学), 2016, 26(3): 172-174, 178.
[9] SUN L F, LIU L W, LV J L, et al. HPLC analysis of flavonoids from Reed leaves [J]. Food Sci(食品科学), 2011, 32(10): 241-245.
[10] VAN D W B, KOEK G H, BAST A, et al. The potential of flavonoids in the treatment of non-alcoholic fatty liver disease [J]. Crit RevFoodSci, 2017, 57(4): 834.
[11] MCKAY D L, CHEN C Y O, ZAMPARIELLO C A, et al. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults [J]. Food Chem, 2015, 168(12): 233-240.
[12] LIAO A H, ZHOU G M, CHEN J H, et al. Simultaneous determination of 6 active compounds inRosa laevigataMichx by HPLC combined with ultrasonic-assisted extraction- gradient wavelength [J]. Food Sci(食品科学), 2017, 38(4): 141-145.
[13] 中国药典. 一部[S]. 2015: 附录73-74.
[14] SHI C, GUO D, ZHANG W T, et al. Antimicrobial activity of protocatechuic acid againstCronobacter sakazakii[J]. Mod Food Sci Tech(现代食品科技), 2017(7): 105-111.
[15] ZHANG X L, LI Y C, NIU X H, et al. Effects of protocatechuic acid on antioxidative abilities in the brains of rotenone-induced Parkinson's disease mice [J]. Pro Mod Biomed(现代生物医学进展), 2011, 11(17): 3248-3251.
[16] CHO A S, JEON S M, KIM M J, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice [J]. Food Chem Toxicol Int J Pub Brit Ind Biol Res Ass, 2010, 48(3): 937.
[17] WANG Y H, GAO Y, LI Z, et al. Anti-proliferative and anti-metastatic effects of protocatechuic acid on mouse breast cancer cell line 4t1 [J]. Acta Nutr Sin(营养学报), 2014, 36(1): 53-57.
[18] THUROW T, LEE S O. Effect of chlorogenic acid and neochlorogenic acid on human colon cancer cells [D]. UA: University of Arkansas, 2012.
[19] ZHANG H N, AN C N, XU M, et al. Protocatechuic acid inhibits oxidative damage in PC12 cells [J]. Chin J New Drugs(中国新药杂志), 2014, 23(3): 347-350.
[20] ZHAO J J, DAI X M, QU Y S, et al. Progress in the pharmacodynamics of chlorogenic acid [J]. Chin Wild Plant Resources(中国野生植物资源), 2013, 32(4): 1-5.
[21] YAO Y L, WANG C M. Research progress of improving effect of chlorogenic acid on alzheimer's [J]. Herald Med(医药导报), 2017, 36(11): 1287-1290.
[22] TANWAR B. Flavonoids: Dietary occurrence and health benefits [J]. Spatula Dd, 2012, 2(1): 59-68.
[23] DOWER J I, GELEIJNSE J M, GIJSBERS L, et al. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial [J]. Am J Clin Nut, 2015, 101(5): 914.
[24] BOK S H, JEONG T S, BAE K H, et al. Method for preventing or treating elevated blood lipid level-related diseases by administering rutin and quercetin [M]. US. 2003.
[25] LI H, YANG B, HUANG J, et al. Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting β-catenin signaling pathway [J]. Toxicol Lett, 2013, 220(3): 219-228.
[26] PERK A A, SHATYNSKAMYTSYK I, GER EK Y C, et al. Rutin mediated targeting of signaling machinery in cancer cells [J]. Cancer Cell Int, 2014, 14(1): 124.
[27] YANG N, JIA X B, ZHANG Z H, et al. Advance in studies on anti-cancer activity and mechanism of flavonoids [J]. Chin J Chin Mater Med(中国中药杂志), 2015, 40(3): 373-381.
QuantitativeDetermination of Three Phenolic Acids and Six Flavonoids ofPhragmites Communis(Cav.) Trin. ex Steud from Yuanjiang by HPLC
ZHANG Yulin, ZHAN Jihua, TAN Yang, LI Ling*, PEI Gang*
(School of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, China)
ABSTRACT:OBJECTIVETo establish quantitativedetermination method of the protocatechuic acid, chlorogenic acid, vanillic acid, naringin, rutin, quercetin, luteolin, kaempferol and apigenin inPhragmites communis(Cav.) Trin. ex Steud from Yuanjiang by HPLC.METHODSThe chromatographic separation was performed on a Diamonsil C18column (250 mm× 4.6 mm, 5 μm) kept at 35 ℃, using methanol-0.1% formic acid aqueousas the mobile phase at a flow rate of 1mL·min-1through gradient elution, and the detection wavelengthwas set at dual wavelength(phenolic acids: 283, 327 nm; flavonoids: 283, 335 nm), the injection volume was 10mL.RESULTSThe three phenolic acids and six flavonoids were isolated at 25 min and 50 min, the method exhibited linear range of 14.8-500mg·mL-1(r=0.999 0-0.999 7), the limits of detection(LOD) was 0.006 3-0.053 9 μg·mL-1, the limits of quantification(LOQ) was 0.021 1-0.179 7 μg·mL-1and the recovery rate was 98.73%-102.44%, RSD was 1.27%-2.20%(n=6).CONCLUSIONThe method is accurate, sensitive, reproducible, and can be used to detect and quantitative the nine components inphragmites communis(Cav.) Trin. ex Steud.
Key words:phragmites communis(Cav.) Trin. ex Steud; phenolic acids; flavonoids; HPLC
中图分类号:R917.101
文献标志码:B
文章编号:1007-7693(2018)09-1317-05
DOI:10.13748/j.cnki.issn1007-7693.2018.09.010
引用本文:张雨林, 詹济华, 谭洋, 等. HPLC测定沅江产芦笋中3种酚酸和6种黄酮类成分含量[J]. 中国现代应用药学, 2018, 35(9): 1317-1321.
收稿日期:2017-10-11
基金项目:湖南省芦笋研究开发中心芦笋产业联合研究开发基金
作者简介:张雨林,女,硕士生 Tel: 18774935514 E-mail: zhangyulinymm@foxmail.com
*通信作者:李玲,女,博士,副教授 Tel: 13808472975 E-mail: 444954437@qq.com 裴刚,男,博士,教授 Tel: (0731)88458510 E-mail: peigang@hotmail.com
(本文责编:曹粤锋)