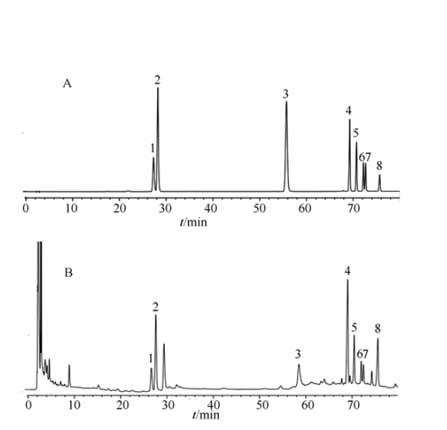
图1 高效液相色谱图
张初瑜1,陈素红2,吴素香1*
(1.浙江中医药大学药学院,杭州 310053;2.浙江工业大学,杭州 310014)
摘要:目的 建立一测多评法(quantitative analysis single-marker,QAMS)测定复方人参片中8种苷类成分的含量。方法 采用Agilent XDB-C18色谱柱(4.6 mm×250 mm,5 µm),流动相水(A)-乙腈(B),梯度洗脱;体积流量1.0 mL·min-1;检测波长203 nm;柱温:25 ℃。以人参皂苷Rb1为内标,计算人参皂苷Rg1、人参皂苷Re、淫羊藿苷、人参皂苷Rc、人参皂苷Rb2、人参皂苷Rb3、人参皂苷Rd的相对校正因子,测定其含量。结果 8种成分在各自范围内线性关系良好(r≥0.999 0),加样回收率为95.6%~104.4%,RSD为1.22%~2.73%,QAMS测定结果与外标法测定结果无显著性差异。结论 该法准确度、灵敏度高、专属性好、操作简单、重复性好。
关键词:高效液相色谱法;苷类;复方人参片;一测多评
复方人参片由人参、淫羊藿等5味中药组成,人参具有大补元气、复脉固脱、补脾益肺、生津养血、安神益智的功能[1],淫羊藿具有补肾阳、强筋骨、祛风湿的功能。复方人参片具有益气、养阴、生津的功效。处方中的人参具有增强免疫、降血糖、减脂、抗衰老、抗疲劳、抗肿瘤等药理作用,人参皂苷是人参发挥药理作用的主要活性成分,而人参皂苷化学结构的多样性是其药理活性多态性的基础[2]。现已发现人参皂苷150多种,主要有人参皂苷Rg1、Rb1、Rc、Rd、Re、Rf、Rg2、Rb3等[3]。淫羊藿具有抗骨质疏松、改善认知、保护心血管、抗肿瘤、抗炎等作用,淫羊藿苷是淫羊藿发挥药理作用的主要活性成分[4]。
含量测定所需对照品稀缺且昂贵,是定量分析中的常见问题,而中成药化学成分的复杂性决定其多成分同时测定作为质控标准更科学、合理。一测多评法(quantitative analysis single-marker,QAMS)是利用待测组分间具有相似的极性、吸收光谱和光谱特性,建立针对各待测组分色谱峰曲线的相对校正因子(relative correct factors,RCF),以减少对照品的使用[5]。Wang等[6]采用QAMS同时测定三七中的人参皂苷Rg1、Rb1、Rg2、Rh1、Rf、Re;三七皂苷R1、R4、Fa、F;Andrey等[7]采用QAMS同时测定人参皂苷Rb1、Rb2、Rb3、Re、Rd、Rg1、Rf、Rg2、Rg3、Rh1、Rh2、R1、CK。但人参复方制剂苷类成分的QAMS相关报道较少,检测成分也较少[8-9]。本实验选用人参皂苷Rg1、Re、Rb1、Rc、Rb2、Rb3、Rd及淫羊藿苷作为复方人参片的质量控制指标,建立QAMS测定8种苷类成分的含量,并与外标法(External standard methed,ESM)测定结果进行比较。
Agilent 1200高效液相色谱仪(美国Agilent公司,包括G1311A四元泵、G1322A在线脱气机、G1329A自动进样器、G1316A柱温箱);Agilent Chemstation色谱工作站;KQ-500DE型数控超声波清洗器(昆山市超声仪器有限公司);Millipore超纯水系统(美国Millipore 公司)。
复方人参片(批号:20160915-1;20160915-2;20160915-3)自制。对照品:人参皂苷Rb2(批号:P09M8F35575;纯度≥98%);人参皂苷Rb3(批号:Z25N7X25518;纯度≥97%)、人参皂苷Rc(批号:P24F8F30057;纯度≥98%)、人参皂苷Rg1(批号:Z26S7X21730;纯度≥98%)、人参皂苷Rb1(批号:Z06M8L30693;纯度≥98%)和淫羊藿苷(批号:T09N6B5664;纯度≥98%)均由上海源叶所提供。人参皂苷Re(中国食品药品检定研究院,批号:110754-201525;纯度:97.4)。人参皂苷Rd(昆明风山渐医药研究有限公司,批号:06031810;纯度≥98%);乙腈为色谱纯,其他试剂为分析纯,水为超纯水。
以十八烷基硅烷键合硅胶为填充剂;流动相为乙腈(A)-水(B);梯度洗脱程序(0~20 min,20%A;20~30 min,20%→25%;30~40 min,25%→21 %;40~45 min,21 %A;45~70 min,21%→35%A;70~ 75 min,35%A;75~85 min,35%→45%A;85~95 min,45%→60%A;95~100 min,60%A);检测波长203 nm;理论板数按人参皂苷Rb1峰计算应≥6 000。流速1.0 min·mL-1;柱温25 ℃。色谱图见图1。
精密称取人参皂苷Rg1、Re、Rb1、Rc、Rb2、Rb3、Rd、淫羊藿苷对照品,加甲醇制成每1 mL分别含1.426,4.286,2.926,1.572,0.86,0.866,0.674,0.614 mg的混合溶液,摇匀,即得。
将复方人参片研细,取粉末约5 g,精密称定,置于索氏提取器中,加石油醚加热回流5 h,弃去石油醚液,药渣挥干溶剂,连同滤纸筒移入150 mL具塞锥形瓶中,加入水饱和正丁醇50 mL,密塞,放置过夜,超声处理(功率500 W,频率40 khz)1 h,滤过,滤液置蒸发皿中蒸干,残渣加甲醇溶解并转移至5 mL量瓶中,加甲醇稀释至刻度,摇匀,滤过,取续滤液,即得。

图1 高效液相色谱图
A-对照品;B-供试品;1-人参皂苷Rg1;2-人参皂苷Re;3-淫羊藿苷;4-人参皂苷Rb1;5-人参皂苷Rc;6-人参皂苷Rb2;7-人参皂苷Rb3;8-人参皂苷Rd。
Fig. 1 HPLC chromatograms
-standara solution; B-sample solution; 1-ginsenoside Rg1; 2-ginsenoside Re; 3-icarrin; 4-ginsenoside Rb1; 5-ginsenoside Rc; 6-ginsenoside Rb2; 7-ginsenoside Rb3; 8-ginsenoside Rd.
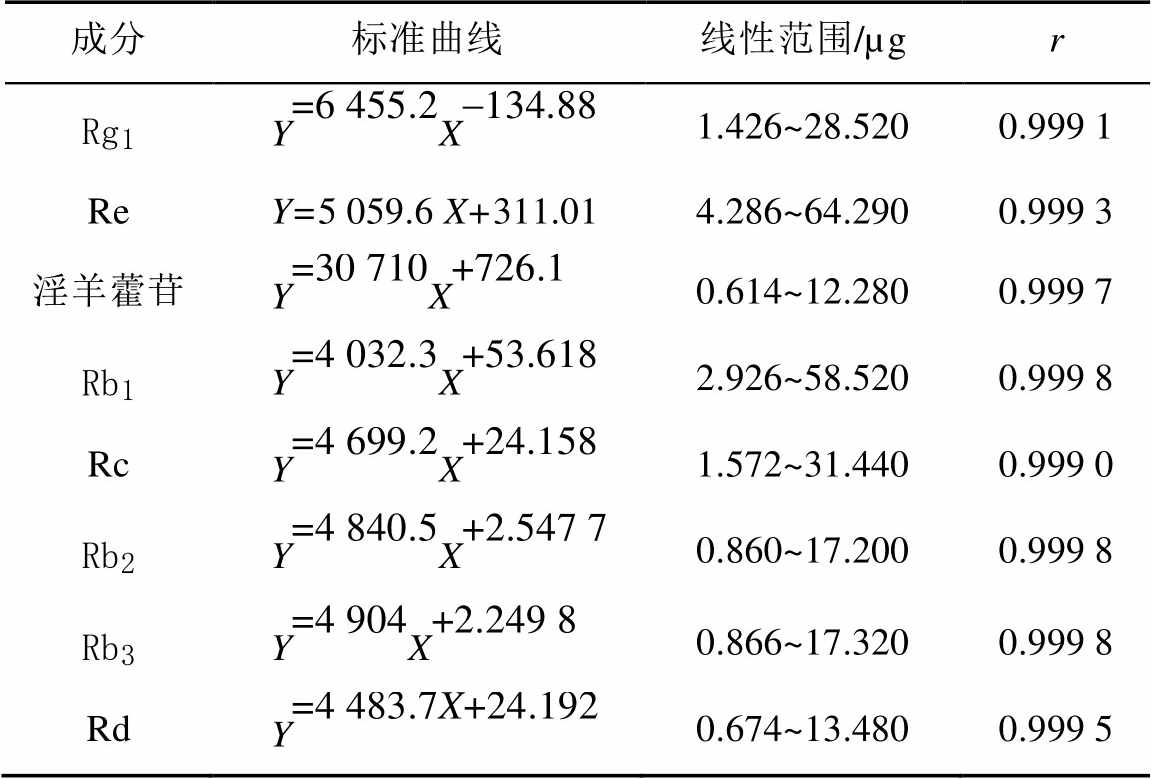
2.4.1 线性关系考察 将混合对照品溶液依次稀释1,2,4,8,10倍,精密吸取混合对照溶液及稀释液各10 µL注入高效液相色谱仪测定。以对照品进样量(mg)为横坐标(X),峰面积为纵坐标(Y),用最小二乘法进行线性回归,得到各个成分的回归方程、线性范围。结果表明,8个成分都具有良好的线性关系,结果见表1。
2.4.2 仪器精密度试验 取同一供试品溶液,按“2.1”项下色谱条件重复进样6次,测得人参皂苷Rg1、Re、Rb1、Rc、Rb2、Rb3、Rd、淫羊藿苷峰面积RSD值分别为0.86%,0.99%,0.69%,2.34%,2.79%,2.89%,1.17%,2.99%。表明仪器精密度良好。
表1 复方人参片中8种成分的标准曲线、线性范围
Tab. 1 The standard’s calibration curves, linear range of eight components in Compound Ginseng tablets
2.4.3 稳定性试验 取同一份供试品溶液,按“2.1”项下色谱条件分别在0,2,4,8,12,24 h进样,测定人参皂苷Rg1、Re、Rb1、Rc、Rb2、Rb3、Rd、淫羊藿苷峰面积,计算其RSD分别为1.17%,1.83%,1.84%,2.78%,2.53%,2.51%,1.72%,1.95%。表明供试品溶液在24 h内稳定性良好。
2.4.4 重复性试验 按“2.3”项下方法平行制备供试品6份,测定,人参皂苷Rg1、Re、Rb1、Rc、Rb2、Rb3、Rd、淫羊藿苷的平均含量分别为1.155 2,3.916 9,6.007 9,1.863 3,0.734 9,0.665 7,2.647 0,0.187 6 μg·g-1;RSD分别为4.16%,2.19%,1.52%,2.62%,1.95%,2.00%,2.12%,2.46%。该方法重复性良好。
2.4.5 加样回收率 精密称取已知含量的复方人参片样品6份,分别精密加入混合对照品溶液适量,按供试品溶液的制备方法制备并测定,计算加样回收率及RSD。结果见表2。
表2 加样回收率试验结果(n=6)
Tab. 2 Results of recovery test(n=6)

2.5.1 校正因子的计算法 按线性关系考察项下的试验方法,通过公式f=fi/fs=(Ai/ci)/(As/Cs)[Ai为待测成分的峰面积,Ci为待测成分的浓度(µg·µL-1);As为内参物s的峰面积,Cs为内参物s的浓度(µg·µL-1)],分别计算人参皂苷Rg1、Rb1为内参物时,其他成分的相对校正因子,并将结果进行比较。结果表明,人参皂苷Rb1为内参物比人参皂苷Rg1为内参物重复性高,结果见表3。因此,本试验选用人参皂苷Rb1为内参物。
2.5.2 待测色谱峰的定位 在一测多评的运用中,能否在不同色谱体系中对待测组分的色谱峰进行准确定位是关键。目前常用的色谱峰定位方法有相对保留值法、保留时间差、时间校正法、对照提取物法等。本试验发现用保留时间差定位人参皂苷Rg1、Re、淫羊藿苷峰,相对保留值法定位人参皂苷Rb1、Rc、Rb2、Rb3、Rd峰,其待测成分相对保留时间与理论相对保留值的相对标准偏差值小,因此采用二者相结合的方法同时定位峰,结果见表4。
表3 人参皂苷Rg1、Rb1作为内参物相对校正因子的测定结果比较
Tab. 3 Comparison of RCFs used ginsenoside Rg1, Rb1 as the internal reference substance

表4 人参皂苷Rb1作为内参物时相对保留值与保留时间差的测定结果
Tab. 4 The results of retention time and relative relention value used ginsenoside Rb1 as the internal reference substance
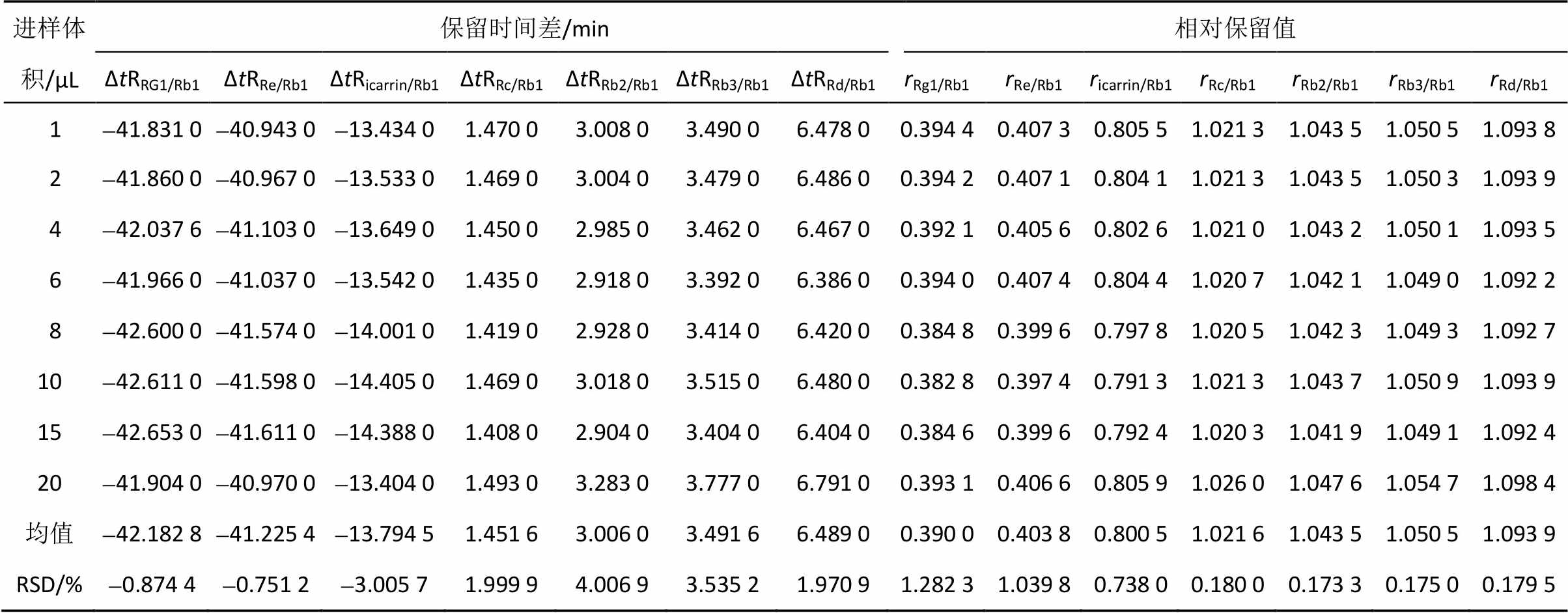
保留时间差法:待测组分(i)与内参物(s)间保留时间的差值,计算公式:ΔtRi/s=tRi-tRs。
相对保留值:待测组分(i)与内参物(s)间保留时间的比值,计算公式:ri/s=ri/rs。
2.6.1 柱温对相对校正因子、保留时间差及相对保留值的影响 按“2.1”项下色谱条件分别在25,30,35,40 ℃的柱温条件下检测各待测成分的相对校正因子、保留时间差及相对保留值,结果见表5~6。
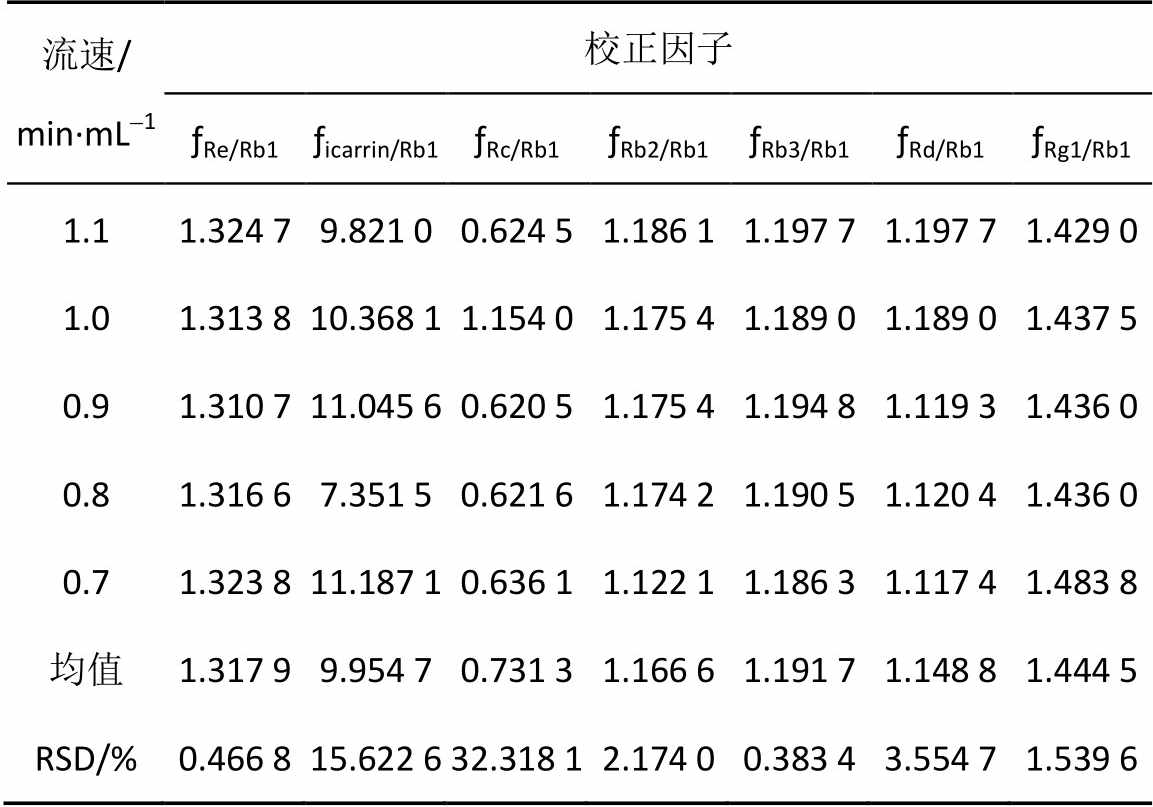
2.6.2 流速对相对校正因子、保留时间差及相对保留值的影响 按“2.1”项下色谱条件,分别在0.7,0.8,0.9,1.1 min·mL-1流速条件下检测各待测成分的相对校正因子、保留时间差及相对保留值,结果见表7~8。
表5 不同柱温下的相对保留值和保留时间差
Tab. 5 Differences in retention time and relative relention value determined on different column temperature

表6 不同柱温下的相对校正因子
Tab. 6 RCFSdetermined on different columns temperature

2.6.3 高效液相色谱仪、色谱柱对相对校正因子、保留时间差及相对保留值的影响 按“2.1”项下色谱条件,分别检测各待测成分在两台不同安捷伦1200高效液相色谱仪及4根不同色谱柱Agilent XDB-C18(4.6 mm×250 mm,5 µm)、Agilent SB-C18(4.6 mm×250 mm,5 µm)、Welch XDB-C18 (4.6 mm×250 mm,5 µm)、Hypersil ODS-SP (4.6 mm×250 mm,5 µm)的相对校正因子、保留时间差及相对保留值,结果见9~10。
表7 不同流速下的相对保留值和保留时间
Tab. 7 Differences in retention time and relative relention value determined on different velocity of flow
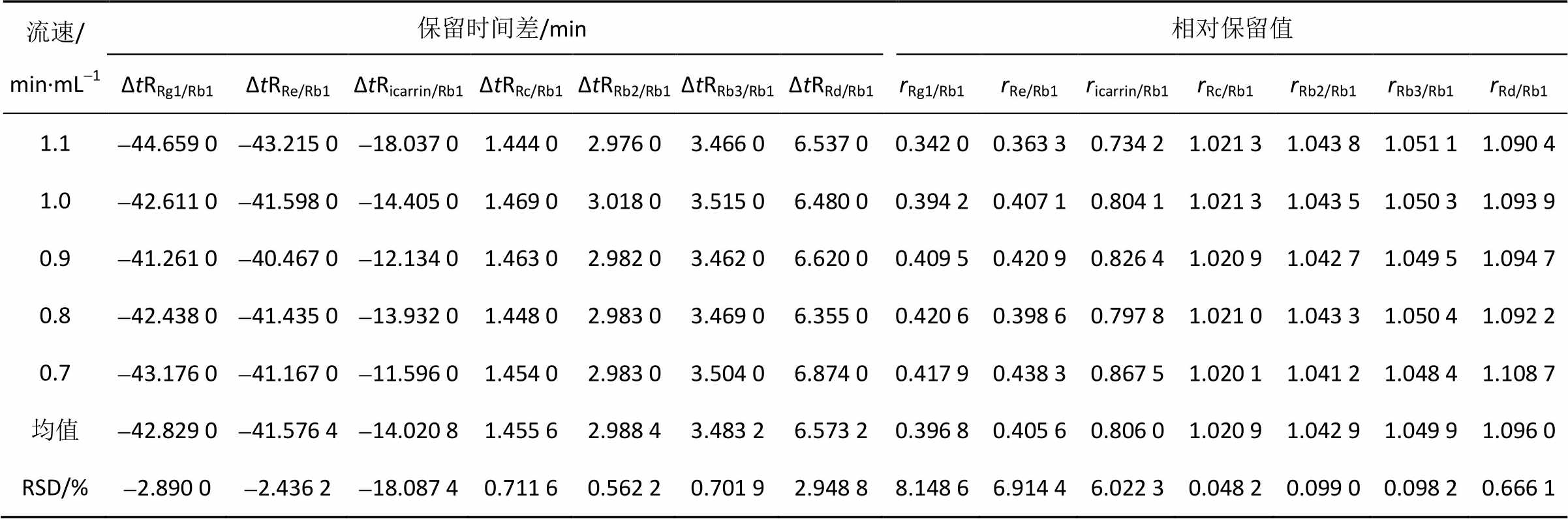
表8 不同流速下的校正因子
Tab. 8 RCFs determined on different velocity of flow
按“2.1”项下色谱条件,分别采用QAMS和ESM对复方人参片的待测成分进行定位,测定,比较,结果见表11。
ESM测定人参皂苷Rg1、Re、Rb1、Rc、Rb2、Rb3、Rd、淫羊藿苷含量的实测值,与QAMS计算值比较,经t检验,P值均>0.05,表明QAMS与ESM测定的结果无显著性差异;QAMS与ESM进行定位,并经t检验,结果发现除淫羊藿苷和人参皂苷Rd外,其他成分P>0.05。结果见表11。
表9 不同高效液相色谱仪、色谱柱下的校正因子
Tab. 9 RCFs determined on different HPLC instruments and columns

表10 不同高效液相色谱仪、色谱柱下的相对保留值和保留时间差
Tab. 10 Differences in retention time and relative relention value determined on different HPLC instrumentsand columns
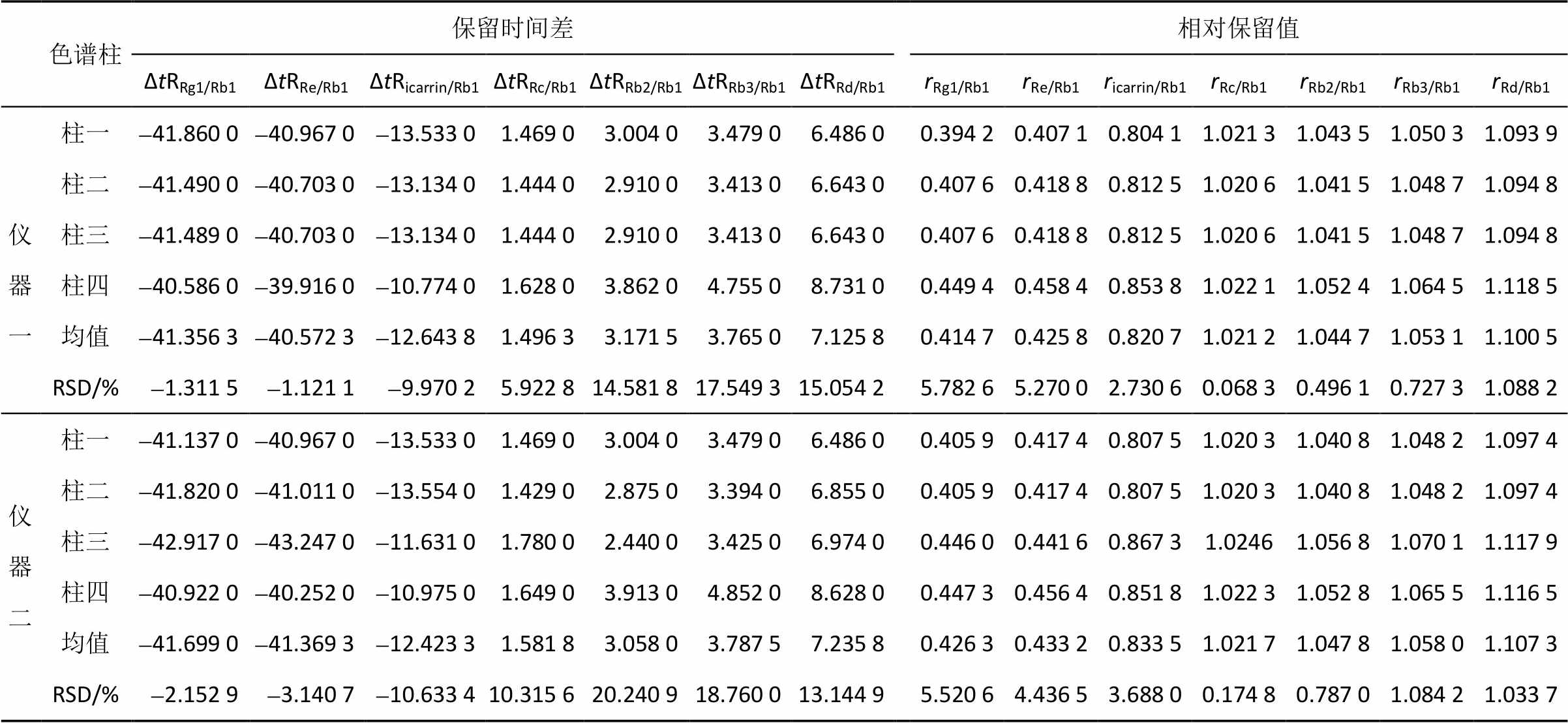
表11 QAMS与ESM含量测定结果(n=7)
Tab. 11 Cmparison of result in Compound Ginseng Tablets by two methods(n=7)

极性皂苷和弱极性皂苷在低波长检测时灵敏度及选择性低,基线波动幅度大。为建立稳定的复方人参片色谱方法,应注意流动相的截止波长以及流动相的均匀性,避免短时间内流动相比例变化幅度过大,不适宜pH值,长时间高比例水相或磷酸分析导致色谱柱柱效降低[10-11],因此本试验将流动相的起始比例调整为乙腈-水(20∶80)。人参皂苷测定多采用蒸散检测器(ELSD)或可变波长检测器(VWD)扫描检测器[12-13],本实验采用二极管阵列检测器较ELSD具普遍性,较VWD可从保留时间与三维图谱同时确定目标成分,防止复方制剂的其他成分对测定的干扰,适用于各类基础实验室对苷类成分的测定,且色谱条件对色谱柱选择范围较宽。本实验考察国产乙腈和进口乙腈对基线波动的影响,结果显示二者无明显差异。
流动相梯度洗脱程序对色谱峰具有明显影响,柱温及流速也对色谱峰分离效果具有重要影响。通过比较待测组分的保留时间、灵敏度及分离度,低流速主要有助于人参皂苷Rg1与Re的分离,同时延长了分析时间,柱温是影响人参皂苷Rb1、Rc、Rb2、Rb3分离的重要因素,高柱温有利于Rb1、Rc、Rb2、Rb3的分离,不利于人参皂Rg1与Re的分离。
人参皂苷Rg1、Re、Rb1、Rc、Rb2、Rb3、Rd、淫羊藿苷在不同色谱柱及仪器的出峰顺序一致,分离度、对称因子均符合系统适用性要求;QAMS与ESM含量测定的结果无显著性差异。本试验采用保留时间差与相对保留值相结合的方法对色谱峰进行定位,除淫羊藿苷和人参皂苷Rd外,其他成分的色谱峰与ESM测得值无显著性差异,可能原因是淫羊藿苷和人参皂苷Rd含量较低。提高人参皂苷Rb1、Rb2、Rb3峰的响应值可以提高方法学的线性、精密度、准确性、重复现性。因此可以通过调节复方人参片样品溶液的进样量以提高系统适应性。
中药制剂具有多成分多靶点的作用特点,人参皂苷Rg1、Re、Rb1、Rc、Rb2、Rb3、Rd、淫羊藿苷是复方人参片中特征活性成分,且与药效具有相关性,是评价片剂质量的重要指标。因人参皂苷Rg1、Rb1廉价易得,所以预实验选用两者作为内参物,并比较其相对校正因子的RSD值,相较于文献中多以人参皂苷Rg1、Re作为内参物[8,14],人参皂苷Rb1作为内参物重复性较好[15],且其相对保留值适中,在203 nm波长处的光谱信号强,与相邻待测峰的分离度>1.5。因此本试验选用人参皂苷Rb1作为内参物。
综上所述,本研究建立了一个快速、准确、耐用性、实用性较好的复方人参片多种苷类成分的一测多评定量方法。有利于实现以人参皂苷Rb1为内参物,通过计算人参皂苷Rg1、Re、Rb1、Rc、Rb2、Rb3、Rd、淫羊藿苷相对校正因子及保留时间差、相对保留值,且避免复方制剂中的不同中药需用不同色谱条件操作的复杂性,最后实现复方人参片多成分、多药效成分的质量评价。
REFERENCES
[1] 中国药典. 一部[S]. 2015: 附录 8-9.
[2] Attele A S, Wu J A, Yuan C S. Ginseng pharmacology: multiple constituents and multiple actions [J]. Biochem Pharmacol, 1999, 58(11): 1685- 1693.
[3] Zhang J J, He S, Zhang L, et al. Comprehensive characterization for ginsenosides biosynthesis in Ginseng Root by integration analysis of chemical and transcriptome [J]. Molecules, 2017, 22(6): 889.
[4] Wu H, Lien E J, Lien L L. Chemical and pharmacological investigations of Epimediumspecies: a survey [J]. Prog Drug Res, 2003(60): 1- 57.
[5] Cui L L, Zhang Y Y, Shao W, et al. Analysis of the HPLC fingerprint and QAMS from Pyrrosia species [J]. Industr Crops Prod, 2016(85): 29- 37.
[6] Wang C Q, Jia X H, Zhu S, et al. A systematic study on the influencing parameters and improvement of quantitative analysis of multi-component with single marker method using notoginseng as research subject [J]. Talanta, 2015(134): 587- 595.
[7] Stavrianidi A, Stekolshchikova E, Porotova A, et al. Combination of HPLC-MS and QAMS as a new analytical approach for determination of saponins in ginseng containing products [J]. J Pharm Biomed Anal, 2016(132): 87-92.
[8] Zhang M L, Cai G Z, Song Y, et al. Study on the determination of saponins in Ginseng Folium medicinal materials, extracts and preparations of saponins by QAMS [J]. Chin J Pharm Anal(药物分析杂志), 2015, 35(6): 997-1001.
[9] Jin R T, Yang S D, Fu J, et al. Determination of eight components in Qinbai Pingfei granule by quantitative analysis of multi-components with a single-marker [J]. Chin Tradit Herb Drugs(中草药), 2015, 46(24): 3682-3686.
[10] Gao Y, Chen L W, Chen D N, et al. Determination of ginsenoside Rg1, Re and Rb1 in Qixue Shuangbu Tinctura by HPLC [J]. Chin Arch Tradit Chin Med(中华中医药学刊), 2015, 33(9): 2095-2097.
[11] Cao S P, Nie L X, Wang G L, et al. HPLC simultaneous determination of nine ginsenosides in Shenmai injection [J]. Chine J Pharm Anal(药物分析杂志), 2011, 31(3): 476-478.
[12] 陈驰, 关琴笑, 朱冬宁等. 一测多评法测定人参中9种人参皂苷的含量[J]. 中药材, 2017, 40(1): 122-126.
[13] Liu X X, Yang L W, Ou G D, et al. Quantitative analysis of four saponins in compoind Danshen tablets by a QAMS [J]. Chin J New Drug(中国新药杂志), 2014, 23(21): 2561-2567.
[14] Geng Y N, Zhang W X. Simultaneous quantitative determination of ginsenosides Re, Rg1, Rb1 and notoginsenoside R1 in Fufang [J]. Chin J Exp Tradit Med Form(中国实验方剂学杂志), 2015, 21(1): 69-71.
[15] Wang X P, Wang F B, Wang J, et al. Simultaneous determination of four saponins in Tengzhuweikang granules by HPLC [J]. Chin J Mod Appl Pharma(中国现代应用药学), 2017, 34(4): 553-556.
(本文责编:曹粤锋)
ZhangChuyu1, Chen Suhong2, Wu Suxiang1*
(1.School of Pharmacy, Zhejiang Chinese Medical University, Hangzhou 310053, China; 2.Zhejiang University of Technology, Hangzhou 310014, China)
ABSTRACT: OBJECTIVE To establish a quantitative of multi-components by single maker for the content determination of eight glycosides in Compound Ginseng tablets. METHODS The analysis of methanol extract of this drug was performed on a 25 ℃ Agilent XDB-C18 column(4.6 mm×250 mm, 5 µm), with the mobile phase of water(A)-acetonitrile(B) flowing at 1.0 mL·min-1 in a gradient elution manner, and the detection wavelength was set at 203 nm. Ginsenoside Rb1 was used as the internal reference substance, RCFs of ginsenoside Rg1, ginsenoside Re, icarrin, ginsenoside Rc, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rd was calculated. The contents of seven consitituents were determined by both external standard and QAMS. The validity of the QAMS method was evaluated by comparison of their quantitative results of both methods. RESULTS Eight consitituents showed good linear relationships within their own ranges (r≥0.999 0), whose average recoveries were 95.6%-104.4% with the RSDs of 1.22%-2.73%. The results obtained by QAMS method approximated those obtained by external standard method. CONCLUSION The method of QAMS turns out to be simple, sensitive, accurate , specific and reproducible in the quality control of Compound Ginseng tablets.
KEY WORDS: HPLC; glycosides; compound ginseng tablets; QAMS
作者简介:张初瑜,女,硕士生 Tel: 15700068035 E-mail: 544309936@QQ. com
*通信作者:吴素香,女,教授,硕导 Tel:13018913115 E-mail: wsx173@126. com
中图分类号:R284.1;R917.101
文献标志码:B
文章编号:1007-7693(2018)05-0708-07
DOI: 10.13748/j.cnki.issn1007-7693.2018.05.019
引用本文:张初瑜, 陈素红, 吴素香. 一测多评法测定复方人参片中的8种苷类成分[J]. 中国现代应用药学, 2018, 35(5): 708-714.
收稿日期:2017-09-04