
Fig. 11H-NMR spectra of scaffold materials
图1 支架的1H-NMR谱
·论 著·
HUANGFU Mingyi, LI Liming, LIU Huina, GUO Wangwei, GUO Ningning, HAN Min*, GAO Jianqing*
(College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China)
ABSTRACT:OBJECTIVE To fabricate a hydrogel scaffold based on hyaluronic acid and modified with a laminin peptide for the growth of bone marrow mesenchymal stem cells (BMSCs). METHODS The scaffold was prepared by the crosslink between HA and adipic hydrazide and modified by a laminin peptide after freeze-drying method. FTIR and NMR spectroscopy were used to characterize the chemical structure of the scaffold. The surface morphology and internal structure of the scaffold were characterized by scanning electron microscopy (SEM) and mercury intrusion. The adhesion growth of BMSCs on the scaffolds was examined by confocal laser scanning microscope(CLSM). RESULTS The results of1H-NMR and FTIR showed that the scaffold was successfully cross-linked and modified by the peptide. SEM results showed that the scaffolds had a regular three-dimensional network structure. The porosity was 94.02% and the water swelling rate was 4 357.0%. CLSM results showed that BMSCs on the peptide-modified scaffold stretched out more pseudopodia and indicated the adhesion of the stent was significantly enhanced. CONCLUSION The peptide-modified hyaluronic acid scaffold showes a high porosity, a regular three-dimensional network structure, and significantly improve the adhesion of stem cells to scaffolds.
KEYWORDS:hyaluronic acid; hydrogel scaffold; mesenchymal stem cell
In the past decade, cell therapy and tissue engineering have emerged rapidly due to the scarcity of organs for transplantation as well as limitations associated with their transplantation including immune rejection and donor site morbidity[1-3]. These approaches aim to substitute or restore the function of damaged organs with a combination of scaffold,growth factors and cells[4-5]. Many progenitor cells including adult and embryonic stem cells have been explored for cell therapy because of their unique capability to differentiate into various cell types required for tissue regeneration[6-7]. Successful stem cell therapy is contingent on the transplantation of a large and pure cell population. In order for these cells to expand, a proper 3D biomimetic microenvironment is needed[8]. Scaffolds act as aframework for adhesion and proliferation of cells.Biomaterial scaffolds provide structural support for cell growth and guide tissue regeneration. An ideal scaffold should be porous to provide rapid nutrient and oxygen transfer for seeded cells[9]. Scaffolds should also possess interconnected porous structure which facilitates the regeneration of tissues,introduction of vasculature and the exchange of nutrient and waste[10].
Hyaluronic acid (HA) is a linear non-sulfated glycosaminoglycan (GAG) abundantly found in the mammalian extracellular matrix (ECM)[11]. HA has been recognized for multiple roles within the mammalian tissue such as water homeostasis, protein binding within the ECM and the cell cytosol, and steric exclusion of other molecules[12]. It is richly present in the extracellular matrix (ECM) of many tissues, contributing to the mechanical integrity of the network. Because it is biocompatible,biodegradable and non-immunogenic, HA has been seen as an attractive starting material for the construction of desired hydrogels[11,13-14]. HA also affects cell proliferation, differentiation, and motility by controlling the ECM elasticity and stiffness. HA has been used in biomedical applications due to the wide range of obtainable molecular weights, diverse modification chemistry, possibility of enzymatic remodeling in cell culture, and nonadhesive nature toward the cell[11,14]. However, because of this nonadhesive nature, HA based scaffolds do not facilitate necessary adhesion for cellular survival and growth. In native ECM, cells can adhere to the matrix by the reaction between integrin on cell surface and laminin in ECM[15]. The peptide motif PPFLMLLKGSTR is found in the α3 chain of the human laminin-5 and can promote cell adhesion through binding with integrin α3β1 receptor[16]. This peptide has been reported to promote wound healing[17]and cellular adhesion[18]on collagen scaffolds. To our knowledge, there is no understanding about modification of HA-based scaffold using this peptide.
Bone marrow mesenchymal stem cells (BMSCs)are a population of multipotent stem cells. BMSCs are able to undergo multilineage differentiation under particular conditions and secrete varieties of cytokines[19]. BMSCs are considered among the most promising cells for transplantation due to the superior properties including ease of isolation, low immunogenicity and are free of ethical controversy[20-21].BMSCs are widely applied in tissue engineering therapies, such as bone regeneration[22]and also nerve tissue engineering[23]applications. In this study,author fabricated a hydrogel scaffold based on HA and modified the scaffold with the peptide PPFLMLLKGSTR. Prescriptions of the scaffold was screened and optimized to endow the scaffold with a highly porous and regular interconnected structure.BMSCs were embedded into the scaffold to evaluate the adhesive and cellular supporting function of the optimized peptide-modified scaffold. BMSCs exhibited spreading adhesive cellular morphology during three-dimentional culture and were shown adhering and growing in the pores of the scaffold.
HA with a molecular mass of 1.30 MDa (batch number: 1503197) or 2.3 MDa (batch number:1307095) was purchased from Zhengda Freda Co.,(Jinan, China); Adipic acid dihydrazide(ADH, batch number: E1726035), NaIO4(batch number: 20130101)and ethylene glycol (batch number: 20131008) were purchased from Aladdin Chemistry Co., Ltd.(Shanghai, China); N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC, batch number:D1627013) was purchased from Sigma-Aldrich (St.Louis, MO). Peptide (PPFLMLLKGSTR) was purchased from Sangon Biotech (Shanghai) Co., Ltd(Shanghai, China). All other chemicals were analytical grade.
FI-IR spectrometer (Nicolet 8700, Thermo Nicolet, USA); Nuclear Magnetic Resonance Spectrometer (600 MHz, Bruker German); Scanning electron microscope (TM-100, Hitachi, Japan); Auto fine coater (JFC-1600, JEOL, Japan); Mercury Intrusion Porosimeter (Autopore IV9510,Micrometeritics, USA).
1.2.1 Preparation of scaffold Aldehyde groups were introduced into the HA chain following a previously-reported method[24]. Briefly, 100 mg HA was dissolved in 33 mL ddH2O (3 mg·mL-1), and sodium periodate (NaIO4) was added drop-wise with stirring by magnetic stirrer. The reaction was carried out for 2 d at room temperature in the dark. Then,excess ethylene glycol was added and stirred for 1 h to terminate the reaction. After that, the mixture was dialyzed against water for 3 d. After freezing the solution at -40 ℃, the functionalized HA was lyophilized. Adipic dihydrazide HA was generated by the following method[25-26]. Adipic acid dihydrazide was added to 3 mg·mL-1HA, and the pH was adjusted to 4.5. Then, EDC was added and the pH maintained at 4.5. The reaction was stopped by adjusting the pH to 7.0. After that, the mixture was dialyzed against water for at least 3 d. Finally,the product was lyophilized and stored at 4 ℃. 2%HA-CHO and HA-ADH (w/v, PBS, pH 8.0) were mixed in equal volumes. After gelation, the products were lyophilized and stored at -20 ℃. The peptide was introduced to the HA by the reaction which produced Schiff base between the amide group of the peptide and the aldehyde group of HA-CHO. Briefly,20 mg HA-CHO was dissolved in ddH2O(3 mg·mL-1), then 0.5 mg peptide (5 mg·mL-1, H2O)was added to the solution drop-wise. The reaction occurred at room temperature in the dark for 2 h.After that, the mixture was dialyzed against water for 3 d. Finally, the product HA-CHO-pep was lyophilized and stored at 4 ℃. HA-CHO-pep and HA-CHO were dissolved in phosphate buffer saline(2%, w/v, PBS, pH 8.0) in the weight ratio of 1∶2,and then were mixed with 2% HA-ADH in equal volumes. After gelation, the products were lyophilized to form HA-pep scaffold.
1.2.2 Isolation and culture of BMSCs For culture of BMSCs, DMEM supplemented with 10%FBS, L-glutamine, 100 IU·mL-1penicillin, and 100 mg·mL-1streptomycin penicillin as the culture medium. BMSCs were isolated from rat bone marrow as previously described[27]. Femurs and tibias were harvested from the sacrificed rats and the ends of bones were removed. The marrow was flushed out from the bones using culture medium and centrifuged for 5 min at 1 200 rpm to collect the cells. The cells were cultured at 37 ℃ and 5% CO2atmosphere in dishes. Medium was changed every 2 d of culture. Cells of passage two to four were used for experiments.
1.2.3 FTIR and NMR FT-IR was used to detect whether the aldehyde group had been successfully introduced onto the HA in the frequency range of 4 000-400 cm-1(KBr pellet). A dried sample of 2 mg was carefully mixed thoroughly with 300 mg dry potassium bromide (KBr) and pressed into a pellet using a macroKBr die kit. The solid pellets were placed in a magnetic holder and the IR spectra were then analyzed.
The structure of the HA-CHO, HA-CHO-peptide, and HA-ADH were determined by NMR spectroscopy using D2O as solvents.
1.2.4 Water uptake behavior The water uptake behavior of the scaffolds was characterized by immersing them in PBS(pH 7.4) at 37 ℃[28]. The dry scaffolds were weighed(W0), immersed in PBS for 5 h. Scaffolds were taken out at different time intervals, blotted with filter paper to remove excess medium on the surface and weighed (Wt) to measure water uptake. The swelling ratio of the scaffolds was calculated by the following equation:
Water absorption(%)=(Wt-W0)/W0×100%.
1.2.5 Porosity and morphology The structural morphology of different scaffolds was examined by scanning electron microscope at an accelerating voltage of 20 kV. Before observation, thin sections of scaffolds were cut with razor blade, placed on sample stubs and coated with gold by sputter coating using Auto Fine Coater JFC-1600. The average pore dimension of the scaffolds was investigated using a mercury porosimeter[29]. The scaffold pore size and distribution were measured using a capillary flow porometer. All of the calculations were performed using the software from porous materials.
1.2.6 Scanning electron microscopy Lyophilized scaffolds were broken and the fracture was coated with gold for scanning electron microscopy (SEM)observation. For examination of BMSCs in scaffolds,the cells were cultured in scaffolds for 3 d before the BMSC-embedded scaffolds were being washed in PBS. The washed samples were fixed in glutaraldehyde for 2 h and, after washed, in osmium tetroxide for 1 h. After the fixative was washed off,the samples were dehydrated in graded ethanol and then dried. Sample fractures were coated with gold and observed by SEM.
1.2.7 Immunofluorescence staining BMSCs were seeded in scaffolds and cultured for 3 d. After being washed in PBS, samples were fixed in 4%paraformaldehyde for 15 min. After being washed thorough with PBS, samples were cryosectioned into 20 μm slices. The slices were rinsed with 0.1%Triton-X100 (Amresco, Ohio, America) for 10 min for permeabilizing and then with 1% goat serum(Boster) for 20 min for blocking. The samples were incubated with Anti-paxillin Polyclonal IgG (Boster)at 37 ℃ for 1 h. After thoroughly washing, the samples were incubated with Alexa Fluor 594 goat anti-rabbit IgG at 37 ℃ for 1 h. Then after being washed in PBS, samples were incubated with Actin-Tracker Green (Beyotime, China) for 30 min at room temperature for labelling of actin filaments.DAPI (Invitrogen) was used to label cell nucleus through incubation in room temperature for 10 min.
The structural characterization of HA-ADH was confirmed by1H-NMR in D2O to observe functional groups (Fig. 1). The peaks in HA-ADH at 1.9 ppm and 4.0-4.5 ppm correspond to the methyl group(-NHCOCH3-) and methenyl group (-CHOH-) of HA,respectively. And peaks belonging to ADH at 2.11 ppm and 1.46 ppm, were still observed in HA-ADH,which confirmed the formation of HA-ADH conjugation.
For PPFLMLLKGSTR peptide, the signals ranging from 7.0 to 7.5 ppm belong to protons of phenyl rings of the pep, which could still be observed in HA-CHO-pep. The peaks of HA-CHO spectra were also observed in HA-CHO-pep1H-NMR mostly, which indicated that the peptide was introduced to HA-CHO chain (Fig.1).

Fig. 11H-NMR spectra of scaffold materials
图1 支架的1H-NMR谱
The scaffold was formed by cross-linking between HA-ADH and HA-CHO. As the degree of oxidation of HA-CHO influenced the HA scaffold significantly, it was optimized as shown in Tab. 1.
Tab. 1 Various prescription process of scaffold
表1 支架制备的处方
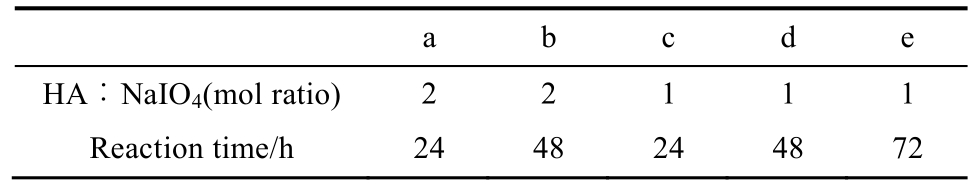
The IR spectrum of HA-CHO showed characteristic absorption peaks at 1 720 cm-1(stretching of C=O in the aldehyde group) in contrast to HA, which confirmed the presence of aldehyde group in HA after being oxidized by NaIO4,as shown in Fig. 2.
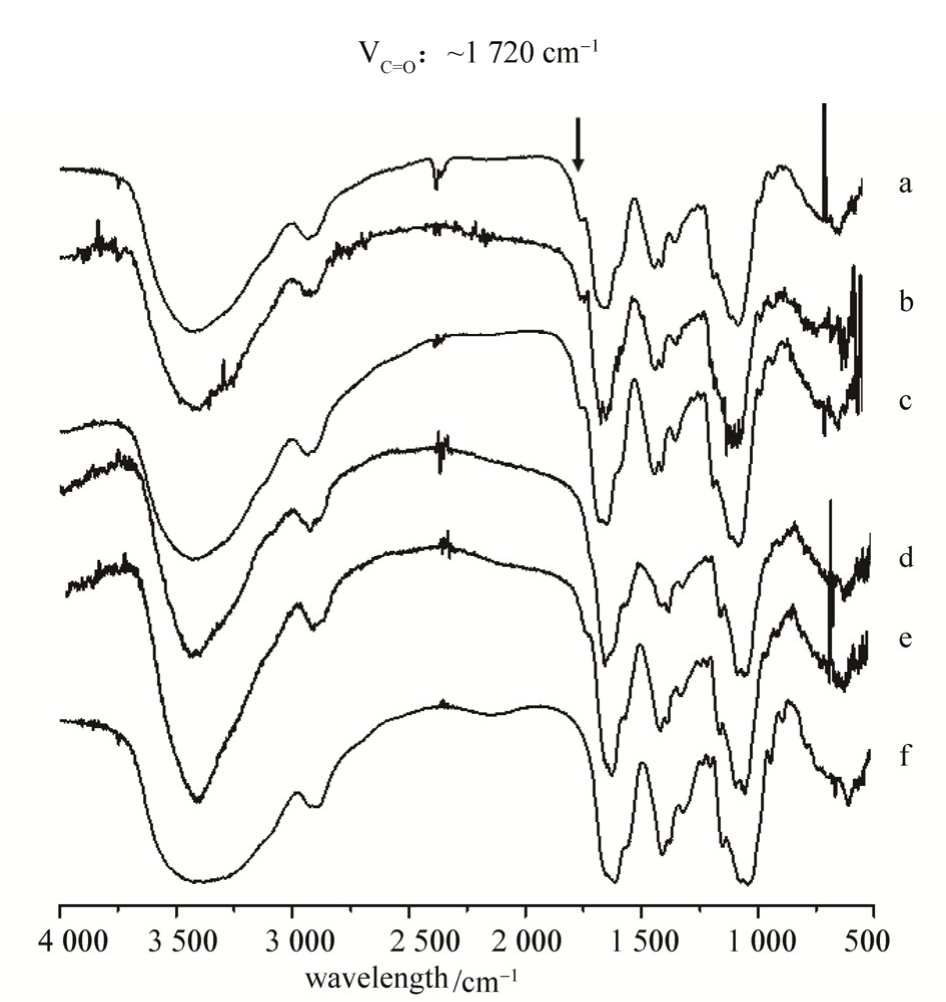
Fig. 2 FTIR spectra of HA-CHO after HA being oxidized by NaIO4to different extent
a-e indicated HA/NaIO4at a ratio of 2, 2, 1, 1, 1, and reaction time was 24, 48, 24, 48, 72 h, respectively; f indicated pure HA powder.
图2 HA支架的红外图谱
a-e表示HA-CHO被不同比例的NaIO4氧化,HA/NaIO4的摩尔比为2∶1, 2∶1, 1∶1, 1∶1, 1∶1,反应时间分别为24, 48, 24, 48,72 h; f为未处理的HA阴性对照。
The scaffolds were screened among various prescriptions (Tab. 1) and optimized for a regularly interconnected highly porous structure. SEM micrographs showed interconnected three-dimentional porous structures of the scaffolds crosslinked by HA-CHO and HA-ADH (Fig. 3). Scaffold of prescription b exhibited the most superior microstructure with regularly connected and uniform pores and was applied for further modification and investigation.
Although HA-based biomaterials had been widely employed for use in tissue engineering scaffolds, they fail to be independently used as bio-scaffolds for cell growth because of poor mechanical properties and a lack of sites for cell recognizing and adhesion[11]. Alginate[30], chitosan[31],collagen[32]and fibrin[33]had been traditionally composited with HA to overcome these shortcomings. However, further study had proved that amino acid, peptide, protein and adhesion molecules modification could more significantly improve cell adhesion and growth on the scaffold[34].
Laminin was a glycol-protein made of α, β and Ƴ peptides, which played an irreplaceable role in cell adhesion, growth, immigration and differentiation. However, its application in tissue engineering scaffold was limited by the large molecular weight which affects the scaffolds properties and the possible immune irritations which come from the protein hydrolysis. Recent study had discovered the crucial peptide sequences of laminin responsible for cell adhesion. These easily modified peptide sequences are stable bothin vivoandin vitrowith small steric hindrance and high tolerance during chemical reactions. PPFLMLLKGSTR peptide was the binding site to integrin α3β1 in laminin-5 α3 chain, which enhances cell adhesion and growth[17],and it was employed to modify the HA scaffold as shown in Fig. 4.

Fig. 3 Micrographs of scaffold with various prescription process of HA-CHO (a-e) and HA powder (f) under SEM
a-e indicated HA/NaIO4at a ratio of 2, 2, 1, 1, 1, and reaction time was 24, 48, 24, 48, 72 h, respectively; f indicated pure HA powder.
图3 不同方法制备的HA支架的扫描电镜图
a-e表示HA-CHO被不同比例的NaIO4氧化,HA/NaIO4的摩尔比为2∶1, 2∶1, 1∶1, 1∶1, 1∶1,反应时间分别为24, 48, 24, 48,72 h; f为未处理的HA阴性对照。
SEM examination on the modified scaffold indicated that peptide modification made no significant influence on the 3D porous structure of scaffold b. The porosity of the HA-pep scaffolds(94.0%) satisfied the requirements for tissue engineering. Pore diameters of the modified scaffold were about 100 μm, which has been reported to be profitable for cellular growth. The results as shown in Fig 4.
Scaffolds should have an interconnected structure for cell infiltration, appropriate porosity and pore dimensions allowing large numbers of seeded cells and effective transport of oxygen and nutrients. SEM images showed highly porous HA-pep scaffolds with interconnected pores ranging from 150 to 300 μm. For example, Murphy CM et al.Research found that scaffolds with pores above 300 μm facilitated both highest cell attachment and proliferation, and were a reduction in cell aggregations that develop along the edges of the scaffolds were seen with larger pores[35].
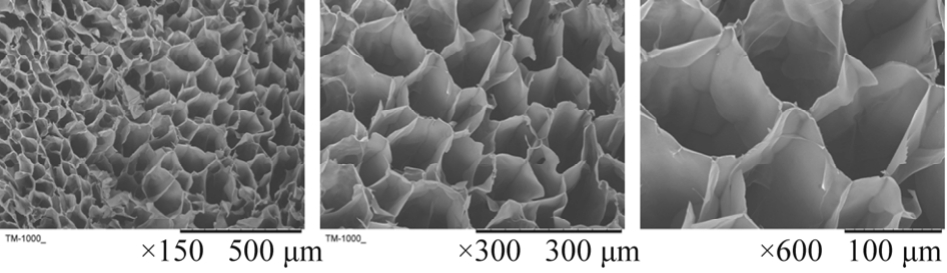
Fig. 4 Micrographs of peptide modified HA scaffold under SEM
图4 多肽修饰的HA支架的扫描电镜图
The ability to swell in aqueous media is an important property of an efficient scaffold, which increases the pore dimension and facilitates in depth filtration of cells in to the scaffolds. Due to the hydrophilic nature of HA and the microstructure of scaffold resulting from the freeze-drying process, all the scaffolds showed good water retention ability. In water uptake study, HA-pep-ADH scaffolds showed rapid swelling up to 1 h, to ~4357% of their weight,and then reached the equilibrium state. The results as shown in Fig 5.
BMSCs, characterized by CD34 and CD73 were cultured in the scaffold for 3 d and observed for cellular adhesion and morphology. The results as shown in Fig 6.
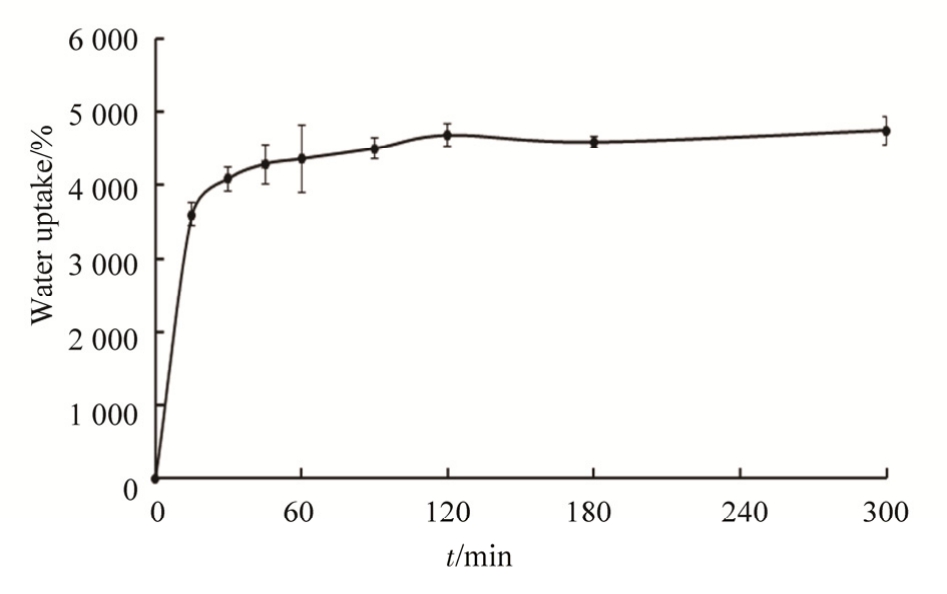
Fig. 5 Water uptake properties of scaffold. Water uptake by the scaffolds (10 mm×10 mm×3 mm thick cylinders) as a function of immersion time in PBS (pH 7.4, 37 ℃). Data are shown as % increase in the scaffold weight, as means ±SD(n=3 per group and time point).
图5 支架的吸水率表征。多肽修饰HA支架在PBS(pH 7.4,37℃)中随时间的吸水率,结果以支架初始质量的百分比表示,n=3。

Fig. 6 Characterization of BMSCs by surface marker expression evaluation on CD34 (left), CD73 (right).
图6 MSC的鉴定和表征。表面抗原CD34(左),CD73(右)表达阳性率的流式细胞仪检测。
A better spreading ability of BMSC could be observed on the peptide-modified HA scaffold (Fig.7B) than that on HA scaffold (Fig. 7A) under scanning electron microscope (SEM).
Furthermore, staining of F-actin and focal adhesion protein paxillin confirmed the cellular adhesion of BMSCs in the scaffold (Fig. 7C and 7D).Light phase in the confocal observation showed the outline of the sectioned scaffold, indicating that the cells lived in the scaffold pores and adhered onto surfaces of the pores in the scaffold. BMSCs exhibited spread cellular morphologies as they adhered and grew in scaffold pores during 3-D culture. And peptide modification significantly promoted adhesion growth of BMSC in scaffold as shown in Fig. 7 under confocal Laser Scanning Microscope(CLSM).3 CONCLUSION
HA hydrogels are one of the most important biopolymer gels used in biomedical applications. In biological applications of HA, fine-tuning mechanical properties of the gels to precise values without significant compositional changes is highly valuable. It is widely recognized in tissue-engineering applications that cell behavior is highly sensitive to the modulus of the gel scaffold.
These results showed that a porous hydrogel based on a laminin peptide and HA was successfully constructed. The hydrogel showed preferable pore size, high swelling behavior and porosity. Author propose that the platform could have potential use in cell proliferation and larger spheroid formation.
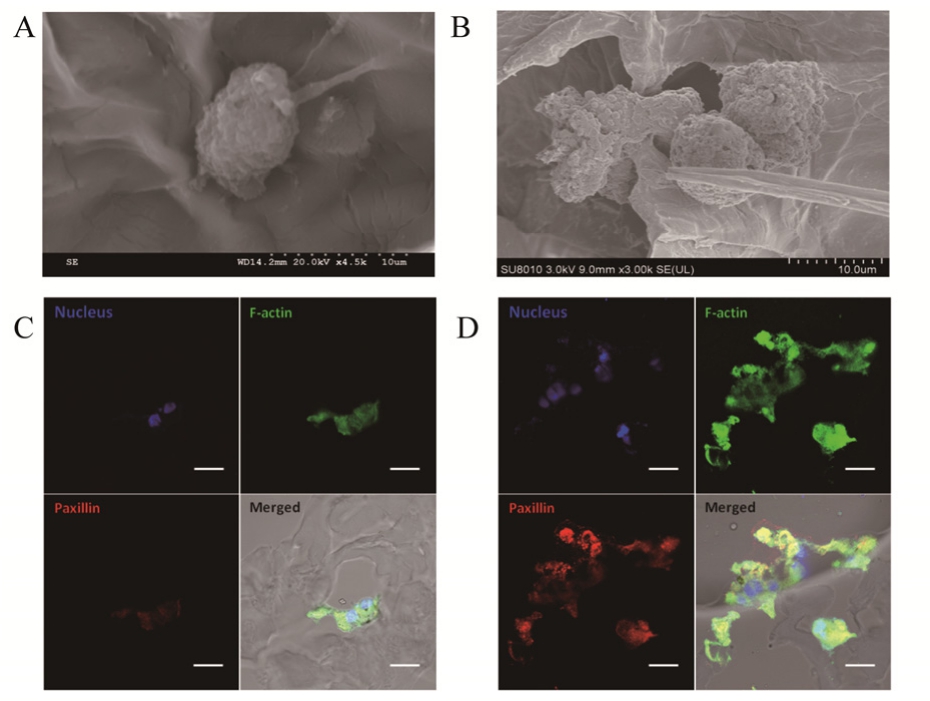
Fig. 7 Micrograph of BMSCs in the HA scaffold (A) and peptide-modified HA scaffold (B) under Scanning Electron Microscope, and staining of focal adhesion protein paxillin(red) and F-actin (green) of BMSCs in the HA scaffold (C)and peptide-modified HA scaffold (D) under CLSM.
图 7 MSC在不同支架上的生长情况。A:透射电镜下BMSC在HA支架上的生长情况;B:透射电镜BMSC在多肽修饰HA支架的生长情况;C:激光共聚焦下BMSC在HA支架的生长情况;D:激光共聚焦下BMSC在多肽修饰HA支架的生长情况。
REFERENCES
[1] CANCEDDA R, DOZIN B, GIANNONI P, et al. Tissue engineering and cell therapy of cartilage and bone [J]. Matrix Biol, 2003, 22(1): 81-91.
[2] RINGE J, KAPS C, BURMESTER G R, et al. Stem cells for regenerative medicine: advances in the engineering of tissues and organs [J]. Naturwissenschaften, 2002, 89(8): 338-351.
[3] FODOR W L. Tissue engineering and cell based therapies,from the bench to the clinic: The potential to replace, repair and regenerate [J]. Reprod Biol Endocrinol, 2003, 13(1): 102.
[4] LEVENBERG S, HUANG N F, Lavik E, et al. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds [J]. Proc Natl Acad Sci, 2003, 100(22): 12741-12746.
[5] LEOR J, AMSALEM Y, COHEN S. Cells, scaffolds, and molecules for myocardial tissue engineering [J]. Pharmacol Ther, 2005, 105(2): 151-163.
[6] BAKSH D, SONG L, TUAN R S. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy [J]. J Cell Mol Med, 2004, 8(3): 301-316.
[7] WOBUS A M, BOHELER K R. Embryonic stem cells:prospects for developmental biology and cell therapy [J].Physiol Rev, 2005, 85(2): 635-678.
[8] RAIC A, RÖDLING L, KALBACHER H, et al. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells [J]. Biomaterial, 2014, 35(3): 929-940.
[9] BOSE S, ROY M, BANDYOPADHYAY A. Recent advances in bone tissue engineering scaffolds [J]. Trends Biotechnol,2012, 30(10): 546-554.
[10] ROUWKEMA J, RIVRON N C, BLITTERSWIJK C A.Vascularization in tissue engineering [J]. Trends Biotechnol,2008, 26(8): 434-441.
[11] LAM J, TRUONG N F, SEGURA T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds [J]. Acta Biomater, 2014, 10(4): 1571-1580.
[12] PRESTON M, SHERMAN L S. Neural stem cell niches:critical roles for the hyaluronan-based extracellular matrix [J].Front Biosci (Schol Ed), 2011(3): 1165-1179.
[13] LEI Y, GOJGINI S, LAM J, et al. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels [J]. Biomaterials, 2010, 32(1):39-47.
[14] SCHANTÉ C E, ZUBER G, HERLIN C, et al. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications [J].Carbohyd Polym, 2011, 85(3): 469-489.
[15] AUMAILLEY M, NURCOMBE V, EDGAR D, et al. The cellular interactions of laminin fragments. Cell adhesion correlates with two fragment-specific high affinity binding sites [J]. J Bio Chem, 1987, 262(24): 11532-11538.
[16] KIM J M, PARK W H, MIN B M. The PPFLMLLKGSTR motif in globular domain 3 of the human laminin-5 alpha3 chain is crucial for integrin alpha3beta1 binding and cell adhesion [J]. Exp Cell Res, 2005, 304(1): 317-327.
[17] DAMODARAN G, TIONG W H, COLLIGHAN R, et al. In vivo effects of tailored laminin-332 α3 conjugated scaffolds enhances wound healing: a histomorphometric analysis [J]. J Biomed Mater Res A, 2013, 101(10): 2788-2795.
[18] DAMODARAN G, COLLIGHAN R, GRIFFIN M, et al.Tailored laminin-332 alpha3 sequence is tethered through an enzymatic linker to a collagen scaffold to promote cellular adhesion [J]. Acta Biomater, 2009, 5(7): 2441-2450.
[19] BAKSH D, YAO R, TUAN R S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow [J]. Stem Cells, 2007, 25(6): 1384-1392.
[20] BIANCO P, RIMINUCCI M, GRONTHOS S, et al. Bone marrow stromal stem cells: Nature, biology, and potential applications [J]. Stem Cells, 2001, 19(3): 180-192.
[21] DERUBEIS A R, CANCEDDA R. Bone marrow stromal cells(BMSCs) in bone engineering: limitations and recent advances[J]. Ann Biomed Eng, 2004, 32(1): 160-165.
[22] ZHANG W, OUYANG H, DASS C R, et al. Current research on pharmacologic and regenerative therapies for osteoarthritis[J]. Bone Res, 2015, 4(4): 185-198.
[23] PRABHAKARAN M P, VENUGOPAL J R,RAMAKRISHNA S. Mesenchymal stem cell differentiation to neuronal cells on electrospun nanofibrous substrates for nerve tissue engineering [J]. Biomaterials, 2009, 30(28): 4996-5003.
[24] DRAYE J P, DELAEY B, VOORDE A V D, et al. In vitro release characteristics of bioactive molecules from dextran dialdehyde cross-linked gelatin hydrogel films [J].Biomaterials, 1998, 19(1-3): 99-107.
[25] YEO Y, HIGHLEY C B, BELLAS E, et al. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model [J].Biomaterials, 2006, 27(27): 4698-4705.
[26] BURDICK J A, PRESTWICH G D. Hyaluronic acid hydrogels for biomedical applications [J]. Adv Mater, 2011, 23(12):41-56.
[27] LI L M, RUAN G X, HUANGFU M Y, et al. ScreenFect A: an efficient and low toxic liposome for gene delivery to mesenchymal stem cells [J]. Int J Pharm, 2015, 488(1/2): 1-11.
[28] CHHABRA H, GUPTA P, VERMA P J, et al. Gelatin-PMVE/MA composite scaffold promotes expansion of embryonic stem cells [J]. Mater Sci Eng C Mater Biol Appl,2014(37): 184-194.
[29] Zan Q. Design and preparation of chitosan; HA composite scaffolds for tissue engineering with long-bone-like structure[J]. Int J Mater Pro Tech, 2010, 37(3/4): 271-279.
[30] KIM Y M, OH S H, CHOI J S, et al. Adipose-derived stem cell-containing hyaluronic acid/alginate hydrogel improves vocal fold wound healing [J]. Laryngoscope, 2014, 124(3):64-72.
[31] ANISHA B S, SANKAR D, MOHANDAS A, et al.Chitosan-hyaluronan/nano chondroitin sulfate ternary composite sponges for medical use [J]. Carbohydr Polym,2013, 92(2): 1470-1476.
[32] CALDERON L, COLLIN E, VELASCOBAYON D, et al.Type II collagen-hyaluronan hydrogel--a step towards a scaffold for intervertebral disc tissue engineering [J]. Eur Cell Mater, 2010, 20(7): 134-148.
[33] SNYDER T N, MADHAVAN K, INTRATOR M, et al.Erratum to: A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair [J]. J Biol Eng, 2014, 8(1): 10.
[34] PARK J, LIM E, BACK S, et al. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive,hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor [J]. J Bio Mater Res Part A, 2010, 93(3): 1091-1099.
[35] MURPHY C M, HAUGH M G, O'BRIEN F J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering [J]. Biomaterials, 2010, 31(3): 461-466.
复合骨髓间充质干细胞的多肽-透明质酸支架的构建与表征
皇甫铭一,李黎明,刘惠娜,郭望葳,郭宁宁,韩旻*,高建青*(浙江大学药学院,杭州 310058)
摘要:目的 采用多肽修饰的透明质酸制备一种复合骨髓间充质干细胞(bone marrow mesenchymal stem cells,BMSCs)的凝胶支架系统。方法 在交联剂作用下,冷冻干燥后,制备得到多肽修饰的透明质酸凝胶支架;使用FTIR和NMR对支架化学结构进行表征;SEM和压汞法对支架表面形态和内部结构进行表征;通过免疫荧光染色和CLSM考察BMSCs在支架上的黏附生长状况。结果1H-NMR和FTIR结果显示,在交联剂存在下,多肽修饰的透明质酸凝胶支架成功制备;SEM观察到凝胶支架具有较规则的三维网状结构。压汞法测定其孔隙率为94.02%,吸水膨胀率为4 357.0%。CLSM结果显示BMSCs在多肽修饰的凝胶支架上伸出较多伪足,具有更强的黏附作用。结论 经多肽修饰的透明质酸凝胶支架具有较高的孔隙率,呈现较规则的三维网状结构,且显著改善了干细胞在凝胶支架上的黏附性,有利于细胞的生长。
关键词:透明质酸;凝胶支架;骨髓间充质干细胞
中图分类号:R943
文献标志码:B
文章编号:1007-7693(2017)12-1647-07
引用本文:皇甫铭一, 李黎明, 刘惠娜, 等. 复合骨髓间充质干细胞的多肽-透明质酸支架的构建与表征[J]. 中国现代应用药学, 2017, 34(12): 1647-1653.
基金项目:国家自然科学基金资助项目(81102392)
作者简介:皇甫铭一,女,硕士生 Tel: 18667039893 E-mail: huangfuy@163.com*
通信作者:韩旻,男,博士,副教授,硕导 Tel:(0571)88208437 E-mail: hanmin@zju.edu.cn 高建青,男,教授,博导 Tel: 13758147918 E-mail: gaojianqing@zju.edu.cn
收稿日期:2017-06-06
(本文责编:李艳芳)
DOI:10.13748/j.cnki.issn1007-7693.2017.12.001